Antiviral RNA Interference against Orsay Virus Is neither Systemic nor Transgenerational in Caenorhabditis elegans
- PMID: 26401037
- PMCID: PMC4645334
- DOI: 10.1128/JVI.03664-14
Antiviral RNA Interference against Orsay Virus Is neither Systemic nor Transgenerational in Caenorhabditis elegans
Abstract
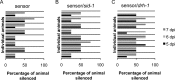
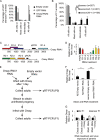
Antiviral RNA-mediated silencing (RNA interference [RNAi]) acts as a powerful innate immunity defense in plants, invertebrates, and mammals. In Caenorhabditis elegans, RNAi is systemic; i.e., RNAi silencing signals can move between cells and tissues. Furthermore, RNAi effects can be inherited transgenerationally and may last for many generations. Neither the biological relevance of systemic RNAi nor transgenerational RNAi is currently understood. Here we examined the role of both pathways in the protection of C. elegans from viral infection. We studied the Orsay virus, a positive-strand RNA virus related to Nodaviridae and the first and only virus known to infect C. elegans. Immunity to Orsay virus infection requires the RNAi pathway. Surprisingly, we found that genes required for systemic or transgenerational RNAi did not have a role in antiviral defense. Furthermore, we found that Orsay virus infection did not elicit a systemic RNAi response even when a target for RNAi was provided by using transgenes. Finally, we show that viral siRNAs, the effectors of RNAi, are not inherited to a level that provides any significant resistance to viral infection in the next generation. We conclude that systemic or transgenerational RNAi does not play a role in the defense against natural Orsay virus infection. Furthermore, our data suggest that there is a qualitative difference between experimental RNAi and antiviral RNAi. Our data are consistent with a model of systemic and transgenerational RNAi that requires a nuclear or germ line component that is lacking in almost all RNA virus infections.
Importance: Since its discovery in Caenorhabditis elegans, RNAi has proven a valuable scientific tool in many organisms. In C. elegans, exogenous RNAi spreads throughout the organism and can be passed between generations; however, there has been controversy as to the endogenous role(s) that the RNAi pathway plays. One endogenous role for which spreading both within the infected organism and between generations would be advantageous is a role in viral defense. In plants, antiviral RNAi is systemic and the spread of RNAi between cells provides protection against subsequent viral infection. Here we investigated this by using the only naturally occurring virus known to infect C. elegans, Orsay virus, and surprisingly found that, in contrast to the exogenous RNAi pathway, the antiviral RNAi response targeted against this virus does not spread systemically throughout the organism and cannot be passed between generations. These results suggest that there are differences between the two pathways that remain to be discovered.
Copyright © 2015 Ashe et al.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

