Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment and Alzheimer's Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer's Disease
- PMID: 26402017
- PMCID: PMC4713833
- DOI: 10.3233/JAD-150336
Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment and Alzheimer's Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer's Disease
Abstract
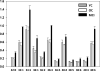
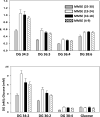
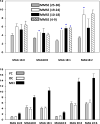
Previous studies have demonstrated augmented levels of diacylglycerols (DAG) in the frontal cortex and plasma of Alzheimer's disease (AD) patients. We extended these findings from non-targeted lipidomics studies to design a lipidomics platform to interrogate DAGs and monoacylglycerols (MAG) in the frontal cortex and plasma of MCI subjects. Control subjects included both aged normal controls and controls with normal cognition, but AD pathology at autopsy, individuals termed non-demented AD neuropathology. DAGs with saturated, unsaturated, and polyunsaturated fatty acid substituents were found to be elevated in MCI frontal cortex and plasma. Tandem mass spectrometry of the DAGs did not reveal any differences in the distributions of the fatty acid substitutions between MCI and control subjects. While triacylglycerols were not altered in MCI subjects there were increases in MAG levels both in the frontal cortex and plasma. In toto, increased levels of DAGs and MAGs appear to occur early in AD pathophysiology and require both further validation in a larger patient cohort and elucidation of the lipidomics alteration(s) that lead to the accumulation of DAGs in MCI subjects.
Keywords: Alzheimer’s disease; diacylglycerols; mild cognitive impairment; monoacylglycerols.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Augmented frontal cortex diacylglycerol levels in Parkinson's disease and Lewy Body Disease.PLoS One. 2018 Mar 7;13(3):e0191815. doi: 10.1371/journal.pone.0191815. eCollection 2018. PLoS One. 2018. PMID: 29513680 Free PMC article.
-
Non-targeted lipidomics of CSF and frontal cortex grey and white matter in control, mild cognitive impairment, and Alzheimer's disease subjects.Acta Neuropsychiatr. 2015 Oct;27(5):270-8. doi: 10.1017/neu.2015.18. Epub 2015 Apr 10. Acta Neuropsychiatr. 2015. PMID: 25858158
-
Frontal Cortex Epigenetic Dysregulation During the Progression of Alzheimer's Disease.J Alzheimers Dis. 2018;62(1):115-131. doi: 10.3233/JAD-171032. J Alzheimers Dis. 2018. PMID: 29439356
-
New diagnostic criteria for Alzheimer's disease and mild cognitive impairment for the practical neurologist.Pract Neurol. 2012 Apr;12(2):88-96. doi: 10.1136/practneurol-2011-000145. Pract Neurol. 2012. PMID: 22450454 Review.
-
Diacylglycerols as biomarkers of sustained immune activation in Proteinopathies associated with dementia.Clin Chim Acta. 2018 Jan;476:107-110. doi: 10.1016/j.cca.2017.11.009. Epub 2017 Nov 13. Clin Chim Acta. 2018. PMID: 29146478 Review.
Cited by
-
Lipid metabolism and Alzheimer's disease: clinical evidence, mechanistic link and therapeutic promise.FEBS J. 2023 Mar;290(6):1420-1453. doi: 10.1111/febs.16344. Epub 2022 Jan 18. FEBS J. 2023. PMID: 34997690 Free PMC article. Review.
-
Alzheimer's disease is an inherent, natural part of human brain aging: an integrated perspective.Free Neuropathol. 2022 Jul 8;3:17. doi: 10.17879/freeneuropathology-2022-3806. eCollection 2022 Jan. Free Neuropathol. 2022. PMID: 37284149 Free PMC article.
-
LPS-induced lipid alterations in microglia revealed by MALDI mass spectrometry-based cell fingerprinting in neuroinflammation studies.Sci Rep. 2022 Feb 21;12(1):2908. doi: 10.1038/s41598-022-06894-1. Sci Rep. 2022. PMID: 35190595 Free PMC article.
-
Augmented frontal cortex diacylglycerol levels in Parkinson's disease and Lewy Body Disease.PLoS One. 2018 Mar 7;13(3):e0191815. doi: 10.1371/journal.pone.0191815. eCollection 2018. PLoS One. 2018. PMID: 29513680 Free PMC article.
-
Lipidomic Alterations in the Cerebral Cortex and White Matter in Sporadic Alzheimer's Disease.Aging Dis. 2023 Oct 1;14(5):1887-1916. doi: 10.14336/AD.2023.0217. Aging Dis. 2023. PMID: 37196109 Free PMC article.
References
-
- Hye A, Riddoch-Contreras J, Baird AL, Ashton NJ, Bazenet C, Leung R, Westman E, Simmons A, Dobson R, Sattlecker M, Lupton M, Lunnon K, Keohane A, Ward M, Pike I, Zucht HD, Pepin D, Zheng W, Tunnicliffe A, Richardson J, Gauthier S, Soininen H, Kłoszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement. 2014;10:799–807. - PMC - PubMed
-
- Wood PL, Phillipps A, Woltjer RL, Kaye JA, Quinn JF. Increased lysophosphatidylethanolamine and diacylglycerol levels in Alzheimer’s disease plasma. JSM Alzheimer’s Disease and Related Dementia. 2014;1:1001.
-
- González-Domínguez R, García-Barrera T, Gómez-Ariza JL. Application of a novel metabolomic approach based on atmospheric pressure photoionization mass spectrometry using flow injection analysis for the study of Alzheimer’s disease. Talanta. 2015;131:480–9. - PubMed
-
- Wood PL, Barnette BL, Kaye JA, Quinn JF, Woltjer RL. Non-targeted lipidomics of CSF and frontal cortex gray and white matter in control, mild cognitive impairment, and Alzheimer’s disease subjects. Acta Neuropsychiatrica 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

