Maintenance Therapy in Ovarian Cancer with Targeted Agents Improves PFS and OS: A Systematic Review and Meta-Analysis
- PMID: 26402447
- PMCID: PMC4581706
- DOI: 10.1371/journal.pone.0139026
Maintenance Therapy in Ovarian Cancer with Targeted Agents Improves PFS and OS: A Systematic Review and Meta-Analysis
Abstract
Background: Maintenance therapy with targeted agents for prolonging remission for ovarian cancer patients remains controversial. As a result, a meta-analysis was conducted to assess the effectiveness and safety of using maintenance therapy with targeted agents for the treatment of ovarian cancer.
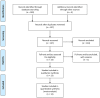
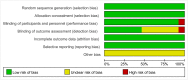
Methods: From inception to January 2015, we searched for randomized, controlled trials (RCTs) using the following databases: PubMed, ScienceDirect, the Cochrane Library, Clinicaltrials.gov and EBSCO. Eligible trials included RCTs that evaluated standard chemotherapy which was either followed or not followed by targeted maintenance in patients with ovarian cancer who had been previously receiving adjunctive treatments, such as cytoreductive surgery and standard chemotherapy. The outcome measures included progression-free survival (PFS), overall survival (OS) and incidence of adverse events.
Results: A total of 13 RCTs, which were published between 2006 and 2014, were found to be in accordance with our inclusion criteria. The primary meta-analysis indicated that both PFS and OS were statistically and significantly improved in the targeted maintenance therapy group as compared to the control group (PFS: HR = 0.84, 95%CI: 0.75 to 0.95, p = 0.001; OS: HR = 0.91, 95%CI: 0.84 to 0.98, p = 0.02). When taking safety into consideration, the use of targeted agents was significantly correlated with increased risks of fatigue, diarrhea, nausea, vomiting, and hypertension. However, no significant differences were found in incidence rates of abdominal pain, constipation or joint pain.
Conclusions: Our results indicate that targeted maintenance therapy clearly improves the survival of ovarian cancer patients but may also increase the incidence of adverse events. Additional randomized, double-blind, placebo-controlled, multicenter investigations will be required on a larger cohort of patients to verify our findings.
Conflict of interest statement
Figures



References
-
- American Cancer Society: Global cancer facts and figures: 2nd edition 2011.
-
- Thigpen T. First-line therapy for ovarian carcinoma: what's next? Cancer investigation. 2004;22(S2):21–8. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63(1):11–30. - PubMed
-
- Lambert H, Rustin G, Gregory W, Nelstrop A. A randomized trial of five versus eight courses of cisplatin or carboplatin in advanced epithelial ovarian carcinoma A North Thames Ovary Group Study. Annals of oncology. 1997;8(4):327–33. - PubMed
-
- Pecorelli S, Favalli G, Gadducci A, Katsaros D, Panici PB, Carpi A, et al. Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the After-6 protocol 1. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(28):4642–8. Epub 2009/08/26. 10.1200/jco.2009.21.9691 . - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

