Meta-Analysis: Reduced Risk of Anxiety with Psychostimulant Treatment in Children with Attention-Deficit/Hyperactivity Disorder
- PMID: 26402485
- PMCID: PMC4617411
- DOI: 10.1089/cap.2015.0075
Meta-Analysis: Reduced Risk of Anxiety with Psychostimulant Treatment in Children with Attention-Deficit/Hyperactivity Disorder
Abstract
Objective: Anxiety is a commonly reported side-effect of psychostimulant treatment. Our goal was to quantify the risk of anxiety as a side effect of psychostimulant treatment for attention-deficit/hyperactivity disorder (ADHD).
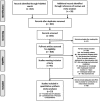
Methods: We conducted a PubMed search to identify all double-blind, randomized, placebo-controlled trials examining the efficacy of psychostimulant medications in the treatment of children with ADHD. We used a fixed-effects meta-analysis to examine the risk ratio of anxiety reported as a side effect in children treated with psychostimulants compared with those treated with placebo. We used stratified subgroup analysis and meta-regression to examine the effects of stimulant type, dosage, duration of use, and trial design on the measured risk of anxiety.
Results: We identified 23 studies involving 2959 children with ADHD for inclusion in our meta-analysis. The risk of anxiety associated with psychostimulant treatment was significantly lower than that experienced with placebo (relative risk [RR] = 0.86 [95% CI: 0.77, 0.95], z = -2.90, p < 0.05). Higher doses of psychostimulants were associated with a reduced measured risk of anxiety of psychostimulants when compared with placebo (β = -0.0039 [95% CI: -0.00718, -0.00064], z = -2.34, p = 0.019).
Conclusions: Meta-analysis suggests that treatment with psychostimulants significantly reduced the risk of anxiety when compared with placebo. This finding does not rule out the possibility that some children experience increased anxiety when treated with psychostimulants, but suggests that those risks are outweighed by the number of children who experience improvement in anxiety symptoms (possibly as a secondary effect of improved control of ADHD symptoms). Clinicians should consider rechallenging children with ADHD who report new-onset or worsening anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.
Figures


References
-
- Abikoff H, McGough J, Vitiello B, McCracken J, Davies M, Walkup J, Riddle M, Oatis M, Greenhill L, Skrobala A, March J, Gammon P, Robinson J, Lazell R, McMahon DJ, Ritz L, Rupp ADHD Anxiety Study Group: Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 44:418–427, 2005 - PubMed
-
- Ahmann PA, Waltonen SJ, Olson KA, Theye FW, Van Erem AJ, LaPlant RJ: Placebo-controlled evaluation of Ritalin side effects. Pediatrics 91:1101–1106, 1993 - PubMed
-
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K: Side effects of methylphenidate in children with attention deficit hyperactivity disorder: A systemic, placebo-controlled evaluation. Pediatrics 86:184–192, 1990 - PubMed
-
- Biederman J, Lopez FA, Boellner SW, Chandler MC: A randomized, double-blind, placebo-controlled, parallel-group study of SLI381 (Adderall XR) in children with attention-deficit/hyperactivity disorder. Pediatrics 110:258–266, 2002 - PubMed
-
- Bilkei–Gorzo A, Gyertyan I, Levay G: mCPP-induced anxiety in the light-dark box in rats–a new method for screening anxiolytic activity. Psychopharmacology 136:291–298, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

