Experimental Malaria in Pregnancy Induces Neurocognitive Injury in Uninfected Offspring via a C5a-C5a Receptor Dependent Pathway
- PMID: 26402732
- PMCID: PMC4581732
- DOI: 10.1371/journal.ppat.1005140
Experimental Malaria in Pregnancy Induces Neurocognitive Injury in Uninfected Offspring via a C5a-C5a Receptor Dependent Pathway
Abstract
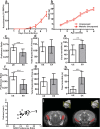
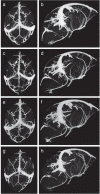
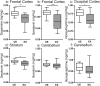
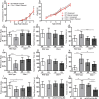
The in utero environment profoundly impacts childhood neurodevelopment and behaviour. A substantial proportion of pregnancies in Africa are at risk of malaria in pregnancy (MIP) however the impact of in utero exposure to MIP on fetal neurodevelopment is unknown. Complement activation, in particular C5a, may contribute to neuropathology and adverse outcomes during MIP. We used an experimental model of MIP and standardized neurocognitive testing, MRI, micro-CT and HPLC analysis of neurotransmitter levels, to test the hypothesis that in utero exposure to malaria alters neurodevelopment through a C5a-C5aR dependent pathway. We show that malaria-exposed offspring have persistent neurocognitive deficits in memory and affective-like behaviour compared to unexposed controls. These deficits were associated with reduced regional brain levels of major biogenic amines and BDNF that were rescued by disruption of C5a-C5aR signaling using genetic and functional approaches. Our results demonstrate that experimental MIP induces neurocognitive deficits in offspring and suggest novel targets for intervention.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, et al. (2003) Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 68: 115–119. - PubMed
-
- Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, et al. (2000) Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol 31: 85–93. - PubMed
-
- Guyatt HL, Snow RW (2001) Malaria in pregnancy as an indirect cause of infant mortality in sub-Saharan Africa. Trans R Soc Trop Med Hyg 95: 569–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

