Subtle Cognitive Decline and Biomarker Staging in Preclinical Alzheimer's Disease
- PMID: 26402771
- PMCID: PMC4634634
- DOI: 10.3233/JAD-150128
Subtle Cognitive Decline and Biomarker Staging in Preclinical Alzheimer's Disease
Abstract
The NIA-AA criteria for "preclinical" Alzheimer's disease (AD) propose a staging method in which AD biomarkers follow an invariable temporal sequence in accordance with the amyloid cascade hypothesis. However, recent findings do not align with the proposed temporal sequence and "subtle cognitive decline," which has not been definitively operationalized, may occur earlier than suggested in preclinical AD. We aimed to define "subtle cognitive decline" using sensitive and reliable neuropsychological tests, and to examine the number and sequence of biomarker abnormalities in the Alzheimer's Disease Neuroimaging Initiative (ADNI). 570 cognitively normal ADNI participants were classified based on NIA-AA criteria and separately based on the number of abnormal biomarkers/cognitive markers associated with preclinical AD that each individual possessed. Results revealed that neurodegeneration alone was 2.5 times more common than amyloidosis alone at baseline. For those who demonstrated only one abnormal biomarker at baseline and later progressed to mild cognitive impairment/AD, neurodegeneration alone was most common, followed by amyloidosis alone or subtle cognitive decline alone, which were equally common. Findings suggest that most individuals do not follow the temporal order proposed by NIA-AA criteria. We provide an operational definition of subtle cognitive decline that captures both cognitive and functional decline. Additionally, we offer a new approach for staging preclinical AD based on number of abnormal biomarkers, without regard to their temporal order of occurrence. This method of characterizing preclinical AD is more parsimonious than the NIA-AA staging system and does not presume that all patients follow a singular invariant expression of the disease.
Keywords: Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s disease; amyloid; biomarkers; cerebrospinal fluid; dementia; neurodegeneration; neuropsychology; preclinical Alzheimer’s disease; subtle cognitive decline.
Figures



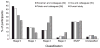
References
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. - PMC - PubMed
-
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. - PMC - PubMed
-
- Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathol. 2013;126:631–641. - PubMed
-
- Drachman DA. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimers Dement. 2014;10:372–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

