Double immunofluorescent staining of rat macrophages in formalin-fixed paraffin-embedded tissue using two monoclonal mouse antibodies
- PMID: 26403093
- PMCID: PMC4758119
- DOI: 10.1007/s00418-015-1364-9
Double immunofluorescent staining of rat macrophages in formalin-fixed paraffin-embedded tissue using two monoclonal mouse antibodies
Abstract
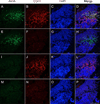
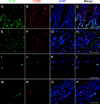
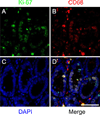
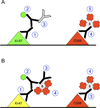
The conventional approach of double immunostaining to visualize more than one protein in tissues or cells using antibodies from two different host species is not always feasible due to limitations with antibody availability. Previously reported methodologies for performing multiple immunostains on the same tissue or cells with antibodies originating from the same species are varied in their complexity, sensitivity, and approach to prevent unwanted interactions between antibodies. In the ever-expanding field of macrophage biology, much more is known about mouse and human macrophages than their rat counterparts. The limited availability of validated and well-characterized monoclonal antibodies from different species is one factor responsible for preventing advances in rat macrophage biology. Here we describe an immunostaining method for identifying and examining rat macrophages that is sufficiently sensitive for use in formalin-fixed paraffin-embedded tissue and that uses only commercially available reagents and antibodies. This method can be used to help characterize both physiological and pathophysiological processes in rat macrophages and can be adapted for use with any two antibodies from the same species of origin as long as one of the antibodies is biotinylated.
Keywords: Biotinylated; Double stain; IHC; Ki-67; Macrophage; Rat.
Conflict of interest statement
CONFLICT OF INTEREST:
The authors declare that they have no conflict of interest.
Figures


References
-
- Boorsma DM. Direct immunoenzyme double staining applicable for monoclonal antibodies. Histochemistry. 1984;80:103–106. - PubMed
-
- Campana D, Janossy G. Leukemia diagnosis and testing of complement-fixing antibodies for bone marrow purging in acute lymphoid leukemia. Blood. 1986;68:1264–1271. - PubMed
-
- Frisch J, et al. Novel multicolor immunofluorescence technique using primary antibodies raised in the same host species. Methods in molecular biology (Clifton, NJ) 2011;717:233–244. - PubMed
-
- Kroeber S, Schomerus C, Korf HW. A specific and sensitive double-immunofluorescence method for the demonstration of S-antigen and serotonin in trout and rat pinealocytes by means of primary antibodies from the same donor species. Histochemistry and cell biology. 1998;109:309–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

