Localisation of 11β-Hydroxysteroid Dehydrogenase Type 2 in Mineralocorticoid Receptor Expressing Magnocellular Neurosecretory Neurones of the Rat Supraoptic and Paraventricular Nuclei
- PMID: 26403275
- PMCID: PMC5019266
- DOI: 10.1111/jne.12325
Localisation of 11β-Hydroxysteroid Dehydrogenase Type 2 in Mineralocorticoid Receptor Expressing Magnocellular Neurosecretory Neurones of the Rat Supraoptic and Paraventricular Nuclei
Abstract
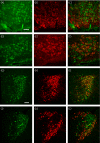
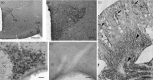
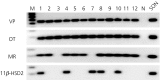
An accumulating body of evidence suggests that the activity of the mineralocorticoid, aldosterone, in the brain via the mineralocorticoid receptor (MR) plays an important role in the regulation of blood pressure. MR was recently found in vasopressin and oxytocin synthesising magnocellular neurosecretory cells (MNCs) in both the paraventricular (PVN) and supraoptic (SON) nuclei in the hypothalamus. Considering the physiological effects of these hormones, MR in these neurones may be an important site mediating the action of aldosterone in blood pressure regulation within the brain. However, aldosterone activation of MR in the hypothalamus remains controversial as a result of the high binding affinity of glucocorticoids to MR at substantially higher concentrations compared to aldosterone. In aldosterone-sensitive epithelia, the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) prevents glucocorticoids from binding to MR by converting glucocorticoids into inactive metabolites. The present study aimed to determine whether 11β-HSD2, which increases aldosterone selectivity, is expressed in MNCs. Specific 11β-HSD2 immunoreactivity was found in the cytoplasm of the MNCs in both the SON and PVN. In addition, double-fluorescence confocal microscopy demonstrated that MR-immunoreactivity and 11β-HSD2-in situ hybridised products are colocalised in MNCs. Lastly, single-cell reverse transcriptase-polymerase chain reaction detected MR and 11β-HSD2 mRNAs from cDNA libraries derived from single identified MNCs. These findings strongly suggest that MNCs in the SON and PVN are aldosterone-sensitive neurones.
Keywords: oxytocin; vasopressin.
© 2015 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Figures



Similar articles
-
11beta-hydroxysteroid dehydrogenase type 2 activity in hypothalamic paraventricular nucleus modulates sympathetic excitation.Hypertension. 2006 Jul;48(1):127-33. doi: 10.1161/01.HYP.0000224296.96235.dd. Epub 2006 May 22. Hypertension. 2006. PMID: 16717146
-
Expression of mineralocorticoid and glucocorticoid receptors in preautonomic neurons of the rat paraventricular nucleus.Am J Physiol Regul Integr Comp Physiol. 2014 Mar 1;306(5):R328-40. doi: 10.1152/ajpregu.00506.2013. Epub 2013 Dec 31. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24381176 Free PMC article.
-
Modulation of oestrogen receptor-beta mRNA expression in rat paraventricular and supraoptic nucleus neurones following adrenal steroid manipulation and hyperosmotic stimulation.J Neuroendocrinol. 2004 May;16(5):472-82. doi: 10.1111/j.1365-2826.2004.01190.x. J Neuroendocrinol. 2004. PMID: 15117341
-
The role of 11beta-hydroxysteroid dehydrogenase in steroid hormone specificity.J Steroid Biochem Mol Biol. 1998 Apr;65(1-6):311-6. doi: 10.1016/s0960-0760(98)00009-0. J Steroid Biochem Mol Biol. 1998. PMID: 9699885 Review.
-
Interactions of mineralocorticoids and glucocorticoids in epithelial target tissues revisited.Steroids. 2009 Jan;74(1):1-6. doi: 10.1016/j.steroids.2008.10.005. Epub 2008 Oct 21. Steroids. 2009. PMID: 19013186 Review.
Cited by
-
Effect of dietary salt intake on epithelial Na+ channels (ENaC) in vasopressin magnocellular neurosecretory neurons in the rat supraoptic nucleus.J Physiol. 2017 Sep 1;595(17):5857-5874. doi: 10.1113/JP274856. Epub 2017 Jul 30. J Physiol. 2017. PMID: 28714095 Free PMC article.
-
Translation of Experimental Findings from Animal to Human Biology: Identification of Neuronal Mineralocorticoid and Glucocorticoid Receptors in a Sectioned Main Nerve Trunk of the Leg.Cells. 2023 Jul 5;12(13):1785. doi: 10.3390/cells12131785. Cells. 2023. PMID: 37443819 Free PMC article.
-
Genome-Wide Analysis of Glucocorticoid-Responsive Transcripts in the Hypothalamic Paraventricular Region of Male Rats.Endocrinology. 2019 Jan 1;160(1):38-54. doi: 10.1210/en.2018-00535. Endocrinology. 2019. PMID: 30364965 Free PMC article.
-
Effect of dietary salt intake on epithelial Na+ channels (ENaCs) in the hypothalamus of Dahl salt-sensitive rats.Physiol Rep. 2018 Aug;6(16):e13838. doi: 10.14814/phy2.13838. Physiol Rep. 2018. PMID: 30156045 Free PMC article.
-
Electrophysiological properties of identified oxytocin and vasopressin neurones.J Neuroendocrinol. 2019 Mar;31(3):e12666. doi: 10.1111/jne.12666. Epub 2019 Feb 14. J Neuroendocrinol. 2019. PMID: 30521104 Free PMC article. Review.
References
-
- Sladek CD. Antidiuretic Hormone: Synthesis and Release. Handbook of physiology, Section 7 2000; The Endocrine System (Endocrine Regulation of Water and Electrolyte Balance). Oxford: Oxford University Press.
-
- Poulain DA, Wakerley JB. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience 1982; 7: 773–808. - PubMed
-
- Verbalis JG. The brain oxytocin receptor(s)? Front Neuroendocrinol 1999; 20: 146–156. - PubMed
-
- Huang W, Lee SL, Sjoquist M. Natriuretic role of endogenous oxytocin in male rats infused with hypertonic NaCl. Am J Physiol 1995; 268: R634–R640. - PubMed
-
- Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocr Rev 1992; 13: 33–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

