Breakdown of albumin and haemalbumin by the cysteine protease interpain A, an albuminase of Prevotella intermedia
- PMID: 26403890
- PMCID: PMC4582931
- DOI: 10.1186/s12866-015-0516-3
Breakdown of albumin and haemalbumin by the cysteine protease interpain A, an albuminase of Prevotella intermedia
Abstract
Background: Prevotella intermedia is a Gram-negative black-pigmenting oral anaerobe associated with periodontitis in humans, and has a haem requirement for growth, survival and virulence. It produces an iron porphyrin-containing pigment comprising monomeric iron (III) protoporphyrin IX (Fe(III)PPIX.OH; haematin). The bacterium expresses a 90-kDa cysteine protease termed interpain A (InpA) which both oxidizes and subsequently degrades haemoglobin, releasing haem. However, it is not known whether the enzyme may play a role in degrading other haem-carrying plasma proteins present in the gingival sulcus or periodontal pocket from which to derive haem. This study evaluated the ability of InpA to degrade apo- and haem-complexed albumin.
Results: Albumin breakdown was examined over a range of pH and in the presence of reducing agent; conditions which prevail in sub- and supra-gingival plaque. InpA digested haemalbumin more efficiently than apoalbumin, especially under reducing conditions at pH 7.5. Under these conditions InpA was able to substantially degrade the albumin component of whole human plasma.
Conclusions: The data point to InpA as an efficient "albuminase" with the ability to degrade the minor fraction of haem-bound albumin in plasma. InpA may thus contribute significantly to haem acquisition by P. intermedia under conditions of low redox potential and higher pH in the inflamed gingival crevice and diseased periodontal pocket where haem availability is tightly controlled by the host.
Figures
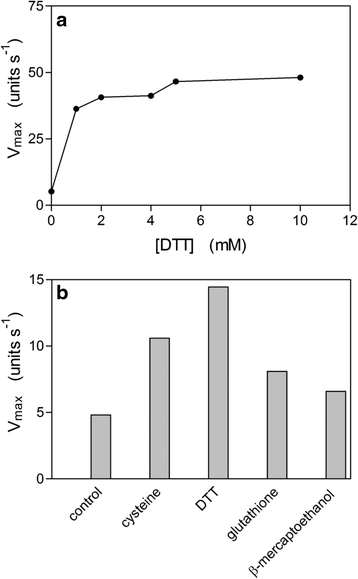

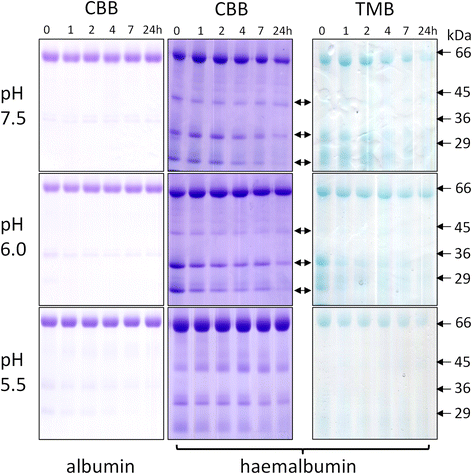

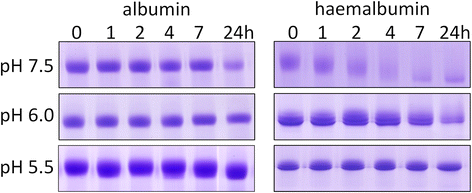
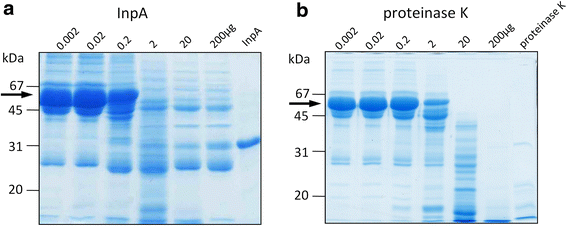
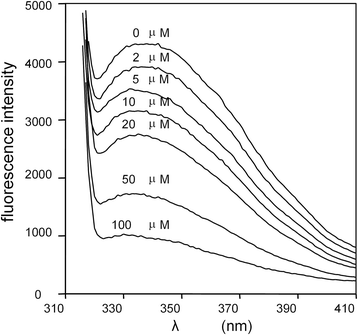
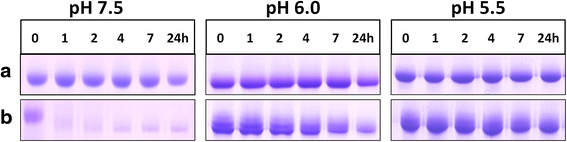

 ) , non-reducing (
) , non-reducing ( ), and to both incubation conditions (
), and to both incubation conditions ( ) are indicated. See text for details
) are indicated. See text for detailsReferences
-
- Smalley JW, Birss AJ, Silver J. The periodontal pathogen Porphyromonas gingivalis harnesses the chemistry of the μ-oxo bishaem of iron protoporphrin IX to protect against hydrogen peroxide. FEMS Microbiol Lett. 2000;183:159–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

