Towards Low Energy Atrial Defibrillation
- PMID: 26404298
- PMCID: PMC4610542
- DOI: 10.3390/s150922378
Towards Low Energy Atrial Defibrillation
Abstract
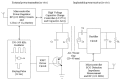
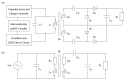
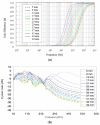
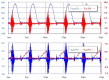
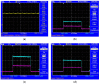
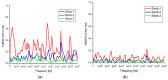
A wireless powered implantable atrial defibrillator consisting of a battery driven hand-held radio frequency (RF) power transmitter (ex vivo) and a passive (battery free) implantable power receiver (in vivo) that enables measurement of the intracardiac impedance (ICI) during internal atrial defibrillation is reported. The architecture is designed to operate in two modes: Cardiac sense mode (power-up, measure the impedance of the cardiac substrate and communicate data to the ex vivo power transmitter) and cardiac shock mode (delivery of a synchronised very low tilt rectilinear electrical shock waveform). An initial prototype was implemented and tested. In low-power (sense) mode, >5 W was delivered across a 2.5 cm air-skin gap to facilitate measurement of the impedance of the cardiac substrate. In high-power (shock) mode, >180 W (delivered as a 12 ms monophasic very-low-tilt-rectilinear (M-VLTR) or as a 12 ms biphasic very-low-tilt-rectilinear (B-VLTR) chronosymmetric (6ms/6ms) amplitude asymmetric (negative phase at 50% magnitude) shock was reliably and repeatedly delivered across the same interface; with >47% DC-to-DC (direct current to direct current) power transfer efficiency at a switching frequency of 185 kHz achieved. In an initial trial of the RF architecture developed, 30 patients with AF were randomised to therapy with an RF generated M-VLTR or B-VLTR shock using a step-up voltage protocol (50-300 V). Mean energy for successful cardioversion was 8.51 J ± 3.16 J. Subsequent analysis revealed that all patients who cardioverted exhibited a significant decrease in ICI between the first and third shocks (5.00 Ω (SD(σ) = 1.62 Ω), p < 0.01) while spectral analysis across frequency also revealed a significant variation in the impedance-amplitude-spectrum-area (IAMSA) within the same patient group (|∆(IAMSAS1-IAMSAS3)[1 Hz - 20 kHz] = 20.82 Ω-Hz (SD(σ) = 10.77 Ω-Hz), p < 0.01); both trends being absent in all patients that failed to cardiovert. Efficient transcutaneous power transfer and sensing of ICI during cardioversion are evidenced as key to the advancement of low-energy atrial defibrillation.
Keywords: RF; battery-free; defibrillator; impedance; implantable; wireless.
Figures






References
-
- Schamroth L. An Introduction to Electrocardiography. 7th ed. Blackwell Scientific Publications; London, UK: 1990.
-
- Fuster V., Rydén L.E., Cannom D.S., Crijns H.J., Curtis A.B., Ellenbogen K.A., Halperin J.L., Le Huezey J.Y., Kay G.N., Lowe J.E., et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651–745. - PubMed
-
- Wann L.S., Curtis A.B., January C.T., Ellenbogen K.A., Lowe J.E., Estes N.A., Page R.L., Ezekowitz M.D., Slotwiner D.J., Jackman W.M., et al. ACCF/AHA/HRS 2011 focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline) J. Am. Coll Cardiol. 2011;57:223–242. doi: 10.1016/j.jacc.2010.10.001. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

