Multi-Facial, Non-Peptidic α-Helix Mimetics
- PMID: 26404384
- PMCID: PMC4588149
- DOI: 10.3390/biology4030540
Multi-Facial, Non-Peptidic α-Helix Mimetics
Abstract
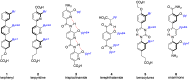
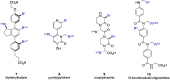
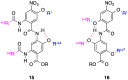
α-Helices often recognize their target proteins at protein-protein interfaces through more than one recognition face. This review describes the state-of-the-art in the design of non-peptidic α-helix mimetics that reproduce functionality from multiple faces of an α-helix.
Keywords: Bak; Bcl-2; Bim; HMD2; amphipathic; cancer; p53; protein–protein interaction; proteomimetic; α-helix mimetic.
Figures
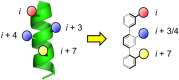

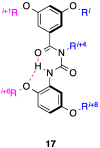
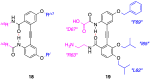
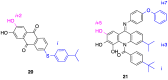
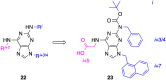
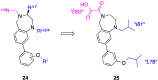
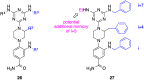
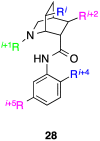
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

