(31)P-MRS of healthy human brain: ATP synthesis, metabolite concentrations, pH, and T1 relaxation times
- PMID: 26404723
- PMCID: PMC4772768
- DOI: 10.1002/nbm.3384
(31)P-MRS of healthy human brain: ATP synthesis, metabolite concentrations, pH, and T1 relaxation times
Abstract
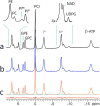
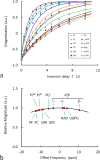
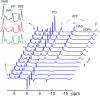
The conventional method for measuring brain ATP synthesis is (31)P saturation transfer (ST), a technique typically dependent on prolonged pre-saturation with γ-ATP. In this study, ATP synthesis rate in resting human brain is evaluated using EBIT (exchange kinetics by band inversion transfer), a technique based on slow recovery of γ-ATP magnetization in the absence of B1 field following co-inversion of PCr and ATP resonances with a short adiabatic pulse. The unidirectional rate constant for the Pi → γ-ATP reaction is 0.21 ± 0.04 s(-1) and the ATP synthesis rate is 9.9 ± 2.1 mmol min(-1) kg(-1) in human brain (n = 12 subjects), consistent with the results by ST. Therefore, EBIT could be a useful alternative to ST in studying brain energy metabolism in normal physiology and under pathological conditions. In addition to ATP synthesis, all detectable (31)P signals are analyzed to determine the brain concentration of phosphorus metabolites, including UDPG at around 10 ppm, a previously reported resonance in liver tissues and now confirmed in human brain. Inversion recovery measurements indicate that UDPG, like its diphosphate analogue NAD, has apparent T1 shorter than that of monophosphates (Pi, PMEs, and PDEs) but longer than that of triphosphate ATP, highlighting the significance of the (31)P-(31)P dipolar mechanism in T1 relaxation of polyphosphates. Another interesting finding is the observation of approximately 40% shorter T1 for intracellular Pi relative to extracellular Pi, attributed to the modulation by the intracellular phosphoryl exchange reaction Pi ↔ γ-ATP. The sufficiently separated intra- and extracellular Pi signals also permit the distinction of pH between intra- and extracellular environments (pH 7.0 versus pH 7.4). In summary, quantitative (31)P MRS in combination with ATP synthesis, pH, and T1 relaxation measurements may offer a promising tool to detect biochemical alterations at early stages of brain dysfunctions and diseases.
Keywords: 31P MRS; ATP; T1 relaxation time; brain metabolism; chemical exchange; inversion transfer; magnetization transfer; pH.
Copyright © 2015 John Wiley & Sons, Ltd.
Figures



References
-
- Du F, Zhu XH, Qiao H, Zhang X, Chen W. Efficient in vivo 31P magnetization transfer approach for noninvasively determining multiple kinetic parameters and metabolic fluxes of ATP metabolism in the human brain. Magn Reson Med. 2007;57(1):103–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

