Microfluidic cytometric analysis of cancer cell transportability and invasiveness
- PMID: 26404901
- PMCID: PMC4585905
- DOI: 10.1038/srep14272
Microfluidic cytometric analysis of cancer cell transportability and invasiveness
Abstract
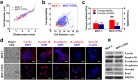
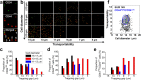
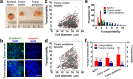
The extensive phenotypic and functional heterogeneity of cancer cells plays an important role in tumor progression and therapeutic resistance. Characterizing this heterogeneity and identifying invasive phenotype may provide possibility to improve chemotherapy treatment. By mimicking cancer cell perfusion through circulatory system in metastasis, we develop a unique microfluidic cytometry (MC) platform to separate cancer cells at high throughput, and further derive a physical parameter 'transportability' to characterize the ability to pass through micro-constrictions. The transportability is determined by cell stiffness and cell-surface frictional property, and can be used to probe tumor heterogeneity, discriminate more invasive phenotypes and correlate with biomarker expressions in breast cancer cells. Decreased cell stiffness and cell-surface frictional force leads to an increase in transportability and may be a feature of invasive cancer cells by promoting cell perfusion through narrow spaces in circulatory system. The MC-Chip provides a promising microfluidic platform for studying cell mechanics and transportability could be used as a novel marker for probing tumor heterogeneity and determining invasive phenotypes.
Figures

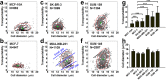
 , where E is Young’s modulus and μ is friction coefficient. (b) The overview shows the cell and buffer inlets on the microfluidic device, scale bar = 1 cm. (c) DLD structure design is shown. Rows of triangular microposts with sides 30 μm in length, 27 μm in height, and separated by 30 μm gaps, are arranged with a tilt angle that gradually increases from the inlet to outlet side of the device, scale bar = 50 μm. (d) Cell size-based separation in the DLD structure is performed by dividing fluid flow into three streams using the microposts. Small cells (blue circles) follow the direction of fluid flow, whereas large cells (red circles) follow the direction of tilt of micropost rows, W = length of the first stream. (e) Stress analyses of trapped cells are performed, N = flow-induced force, arrows indicate the direction of fluid flow, F = compression force from micropost, f1 and f2 = friction forces between cell and micropost.
, where E is Young’s modulus and μ is friction coefficient. (b) The overview shows the cell and buffer inlets on the microfluidic device, scale bar = 1 cm. (c) DLD structure design is shown. Rows of triangular microposts with sides 30 μm in length, 27 μm in height, and separated by 30 μm gaps, are arranged with a tilt angle that gradually increases from the inlet to outlet side of the device, scale bar = 50 μm. (d) Cell size-based separation in the DLD structure is performed by dividing fluid flow into three streams using the microposts. Small cells (blue circles) follow the direction of fluid flow, whereas large cells (red circles) follow the direction of tilt of micropost rows, W = length of the first stream. (e) Stress analyses of trapped cells are performed, N = flow-induced force, arrows indicate the direction of fluid flow, F = compression force from micropost, f1 and f2 = friction forces between cell and micropost.
References
-
- Thiery J. P. & Lim C. T. Tumor dissemination: an EMT affair. Cancer Cell 23, 272–273 (2013). - PubMed
-
- Yilmaz M. & Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metast. Rev. 28, 15–33 (2009). - PubMed
-
- Chaffer C. L. & Weinberg R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011). - PubMed
-
- Di Carlo D. A mechanical biomarker of cell state in medicine. J. Lab. Autom. 17, 32–42 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

