Dermal white adipose tissue: a new component of the thermogenic response
- PMID: 26405076
- PMCID: PMC4617393
- DOI: 10.1194/jlr.R062893
Dermal white adipose tissue: a new component of the thermogenic response
Abstract
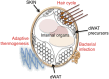
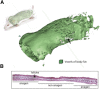
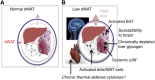
Recent literature suggests that the layer of adipocytes embedded in the skin below the dermis is far from being an inert spacer material. Instead, this layer of dermal white adipose tissue (dWAT) is a regulated lipid layer that comprises a crucial environmental defense. Among all the classes of biological molecules, lipids have the lowest thermal conductance and highest insulation potential. This property can be exploited by mammals to reduce heat loss, suppress brown adipose tissue activation, reduce the activation of thermogenic programs, and increase metabolic efficiency. Furthermore, this layer responds to bacterial challenge to provide a physical barrier and antimicrobial disinfection, and its expansion supports the growth of hair follicles and regenerating skin. In sum, this dWAT layer is a key defensive player with remarkable potential for modifying systemic metabolism, immune function, and physiology. In this review, we discuss the key literature illustrating the properties of this recently recognized adipose depot.
Keywords: adipocytes; antimicrobial; cytokines; diabetes; environmental defense; follicular development; insulation; skin; thermogenesis.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures