The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms
- PMID: 26405100
- PMCID: PMC4698450
- DOI: 10.1152/ajpcell.00156.2015
The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms
Abstract
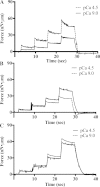
Skeletal muscles present a non-cross-bridge increase in sarcomere stiffness and tension on Ca(2+) activation, referred to as static stiffness and static tension, respectively. It has been hypothesized that this increase in tension is caused by Ca(2+)-dependent changes in the properties of titin molecules. To verify this hypothesis, we investigated the static tension in muscles containing different titin isoforms. Permeabilized myofibrils were isolated from the psoas, soleus, and heart ventricle from the rabbit, and tested in pCa 9.0 and pCa 4.5, before and after extraction of troponin C, thin filaments, and treatment with the actomyosin inhibitor blebbistatin. The myofibrils were tested with stretches of different amplitudes in sarcomere lengths varying between 1.93 and 3.37 μm for the psoas, 2.68 and 4.21 μm for the soleus, and 1.51 and 2.86 μm for the ventricle. Using gel electrophoresis, we confirmed that the three muscles tested have different titin isoforms. The static tension was present in psoas and soleus myofibrils, but not in ventricle myofibrils, and higher in psoas myofibrils than in soleus myofibrils. These results suggest that the increase in the static tension is directly associated with Ca(2+)-dependent change in titin properties and not associated with changes in titin-actin interactions.
Keywords: force enhancement; myofibrils; myosin; static tension.
Copyright © 2016 the American Physiological Society.
Figures


Comment in
-
More roles for the (passive) giant. Focus on "The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms".Am J Physiol Cell Physiol. 2016 Jan 1;310(1):C17-8. doi: 10.1152/ajpcell.00310.2015. Epub 2015 Nov 4. Am J Physiol Cell Physiol. 2016. PMID: 26538091 Free PMC article. No abstract available.
-
Letter to the editor: "Titin-actin interaction: the report of its death was an exaggeration".Am J Physiol Cell Physiol. 2016 Apr 1;310(7):C622. doi: 10.1152/ajpcell.00339.2015. Am J Physiol Cell Physiol. 2016. PMID: 27074703 Free PMC article. No abstract available.
-
Reply to "Letter to the editor: Titin-actin interaction: the report of its death was an exaggeration".Am J Physiol Cell Physiol. 2016 Apr 1;310(7):C623. doi: 10.1152/ajpcell.00365.2015. Am J Physiol Cell Physiol. 2016. PMID: 27074704 No abstract available.
-
Letter to the editor: Comments on Cornachione et al. (2016): "The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms".Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C158-9. doi: 10.1152/ajpcell.00373.2015. Am J Physiol Cell Physiol. 2016. PMID: 27413180 Free PMC article. No abstract available.
-
Reply to "Letter to the editor: Comments on Cornachione et al. (2016): "The increase in non-cross-bridge forces after stretch of activated striated muscle is related to titin isoforms".Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C160-1. doi: 10.1152/ajpcell.00159.2016. Am J Physiol Cell Physiol. 2016. PMID: 27413181 Free PMC article. No abstract available.
References
-
- Bagni MA, Colombini B, Colomo F, Geiger P, Berlinguer PR, Cecchi G. Force response to stretches in activated frog muscle fibres at low tension. Adv Exp Med Biol 538: 429–438, 2003. - PubMed
-
- Bagni MA, Colombini B, Colomo F, Palmini RB, Cecchi G. Non cross-bridge stiffness in skeletal muscle fibres at rest and during activity. Adv Exp Med Biol 565: 141–154, 2005. - PubMed
-
- Bagni MA, Colombini B, Geiger P, Berlinguer PR, Cecchi G. Non-cross-bridge calcium-dependent stiffness in frog muscle fibers. Am J Physiol Cell Physiol 286: C1353–C1357, 2004. - PubMed
-
- Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, Mcnabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. The complete gene sequence of titin, expression of an unusual ∼700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89: 1065–1072, 2001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

