Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients
- PMID: 26405571
- PMCID: PMC4570143
- DOI: 10.1080/2162402X.2015.1019197
Intranodal vaccination with mRNA-optimized dendritic cells in metastatic melanoma patients
Abstract
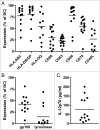
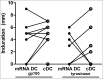
Autologous dendritic cell (DC) therapy is an experimental cellular immunotherapy that is safe and immunogenic in patients with advanced melanoma. In an attempt to further improve the therapeutic responses, we treated 15 patients with melanoma, with autologous monocyte-derived immature DC electroporated with mRNA encoding CD40 ligand (CD40L), CD70 and a constitutively active TLR4 (caTLR4) together with mRNA encoding a tumor-associated antigen (TAA; respectively gp100 or tyrosinase). In addition, DC were pulsed with keyhole limpet hemocyanin (KLH) that served as a control antigen. Production of this DC vaccine with high cellular viability, high expression of co-stimulatory molecules and MHC class I and II and production of IL-12p70, was feasible in all patients. A vaccination cycle consisting of three vaccinations with up to 15×106 DC per vaccination at a biweekly interval, was repeated after 6 and 12 months in the absence of disease progression. mRNA-optimized DC were injected intranodally, because of low CCR7 expression on the DC, and induced de novo immune responses against control antigen. T cell responses against tyrosinase were detected in the skin-test infiltrating lymphocytes (SKIL) of two patients. One mixed tumor response and two durable tumor stabilizations were observed among 8 patients with evaluable disease at baseline. In conclusion, autologous mRNA-optimized DC can be safely administered intranodally to patients with metastatic melanoma but showed limited immunological responses against tyrosinase and gp100.
Keywords: DC vaccines; dendritic cell; immunotherapy; mRNA electroporation; melanoma.
Figures

References
-
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419-26; PMID:17898760; http://dx.doi.org/10.1038/nature06175 - DOI - PubMed
-
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10:475-80; PMID:15122249; http://dx.doi.org/10.1038/nm1039 - DOI - PubMed
-
- Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T et al.. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997; 387:713-7; PMID:9192897; http://dx.doi.org/10.1038/42716 - DOI - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; http://dx.doi.org/10.1038/32588 - DOI - PubMed
-
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999; 5:405-11; PMID:10202929; http://dx.doi.org/10.1038/7403 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
