Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma
- PMID: 26406148
- PMCID: PMC5719487
- DOI: 10.1056/NEJMoa1510665
Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma
Abstract
Background: Nivolumab, a programmed death 1 (PD-1) checkpoint inhibitor, was associated with encouraging overall survival in uncontrolled studies involving previously treated patients with advanced renal-cell carcinoma. This randomized, open-label, phase 3 study compared nivolumab with everolimus in patients with renal-cell carcinoma who had received previous treatment.
Methods: A total of 821 patients with advanced clear-cell renal-cell carcinoma for which they had received previous treatment with one or two regimens of antiangiogenic therapy were randomly assigned (in a 1:1 ratio) to receive 3 mg of nivolumab per kilogram of body weight intravenously every 2 weeks or a 10-mg everolimus tablet orally once daily. The primary end point was overall survival. The secondary end points included the objective response rate and safety.
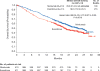
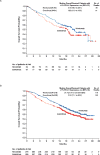
Results: The median overall survival was 25.0 months (95% confidence interval [CI], 21.8 to not estimable) with nivolumab and 19.6 months (95% CI, 17.6 to 23.1) with everolimus. The hazard ratio for death with nivolumab versus everolimus was 0.73 (98.5% CI, 0.57 to 0.93; P=0.002), which met the prespecified criterion for superiority (P≤0.0148). The objective response rate was greater with nivolumab than with everolimus (25% vs. 5%; odds ratio, 5.98 [95% CI, 3.68 to 9.72]; P<0.001). The median progression-free survival was 4.6 months (95% CI, 3.7 to 5.4) with nivolumab and 4.4 months (95% CI, 3.7 to 5.5) with everolimus (hazard ratio, 0.88; 95% CI, 0.75 to 1.03; P=0.11). Grade 3 or 4 treatment-related adverse events occurred in 19% of the patients receiving nivolumab and in 37% of the patients receiving everolimus; the most common event with nivolumab was fatigue (in 2% of the patients), and the most common event with everolimus was anemia (in 8%).
Conclusions: Among patients with previously treated advanced renal-cell carcinoma, overall survival was longer and fewer grade 3 or 4 adverse events occurred with nivolumab than with everolimus. (Funded by Bristol-Myers Squibb; CheckMate 025 ClinicalTrials.gov number, NCT01668784.).
Figures


Comment in
-
Renal-Cell Cancer--Targeting an Immune Checkpoint or Multiple Kinases.N Engl J Med. 2015 Nov 5;373(19):1872-4. doi: 10.1056/NEJMe1511252. Epub 2015 Sep 25. N Engl J Med. 2015. PMID: 26406149 Free PMC article. No abstract available.
-
CheckMate for advanced-stage ccRCC? Nivolumab and cabozantinib aMETEORate poor survival.Nat Rev Urol. 2015 Dec;12(12):651. doi: 10.1038/nrurol.2015.246. Epub 2015 Oct 13. Nat Rev Urol. 2015. PMID: 26458750 No abstract available.
-
Kidney cancer: CheckMate for advanced-stage ccRCC? Nivolumab and cabozantinib aMETEORate poor survival.Nat Rev Clin Oncol. 2015 Nov;12(11):621. doi: 10.1038/nrclinonc.2015.178. Epub 2015 Oct 13. Nat Rev Clin Oncol. 2015. PMID: 26462125 No abstract available.
-
Nivolumab: The New Second Line Treatment for Advanced Renal-cell Carcinoma Commentary on: Nivolumab Versus Everolimus in Advanced Renal-cell Carcinoma.Urology. 2016 Mar;89:8-9. doi: 10.1016/j.urology.2015.12.003. Epub 2015 Dec 23. Urology. 2016. PMID: 26723186 No abstract available.
-
Words of Wisdom. Re: Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma.Eur Urol. 2016 Mar;69(3):538-9. doi: 10.1016/j.eururo.2015.12.025. Eur Urol. 2016. PMID: 26867725 No abstract available.
-
Treatment of Advanced Renal-Cell Carcinoma.N Engl J Med. 2016 Mar 3;374(9):889-90. doi: 10.1056/NEJMc1515613. N Engl J Med. 2016. PMID: 26962911 No abstract available.
-
Treatment of Advanced Renal-Cell Carcinoma.N Engl J Med. 2016 Mar 3;374(9):888. doi: 10.1056/NEJMc1515613. N Engl J Med. 2016. PMID: 26962912 No abstract available.
-
Treatment of Advanced Renal-Cell Carcinoma.N Engl J Med. 2016 Mar 3;374(9):888-9. doi: 10.1056/NEJMc1515613. N Engl J Med. 2016. PMID: 26962913 No abstract available.
-
Words of Wisdom. Re: Nivolumab Versus Everolimus in Advanced Renal-Cell Carcinoma.Eur Urol. 2016 Apr;69(4):753-4. doi: 10.1016/j.eururo.2016.01.018. Epub 2016 Feb 18. Eur Urol. 2016. PMID: 26972496 No abstract available.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Fisher R, Gore M, Larkin J. Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol. 2013;23:38–45. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Kidney cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2015. Version 3.2015. - PubMed
-
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii49–iii56. - PubMed
-
- Afinitor (everolimus) [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corp.; 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
