Value of Routine Dengue Diagnostic Tests in Urine and Saliva Specimens
- PMID: 26406240
- PMCID: PMC4583371
- DOI: 10.1371/journal.pntd.0004100
Value of Routine Dengue Diagnostic Tests in Urine and Saliva Specimens
Abstract
Background: Dengue laboratory diagnosis is essentially based on detection of the virus, its components or antibodies directed against the virus in blood samples. Blood, however, may be difficult to draw in some patients, especially in children, and sampling during outbreak investigations or epidemiological studies may face logistical challenges or limited compliance to invasive procedures from subjects. The aim of this study was to assess the possibility of using saliva and urine samples instead of blood for dengue diagnosis.
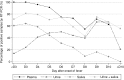
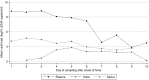
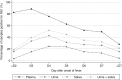
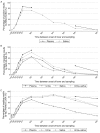
Methodology/principal findings: Serial plasma, urine and saliva samples were collected at several time-points between the day of admission to hospital until three months after the onset of fever in children with confirmed dengue disease. Quantitative RT-PCR, NS1 antigen capture and ELISA serology for anti-DENV antibody (IgG, IgM and IgA) detection were performed in parallel on the three body fluids. RT-PCR and NS1 tests demonstrated an overall sensitivity of 85.4%/63.4%, 41.6%/14.5% and 39%/28.3%, in plasma, urine and saliva specimens, respectively. When urine and saliva samples were collected at the same time-points and tested concurrently, the diagnostic sensitivity of RNA and NS1 detection assays was 69.1% and 34.4%, respectively. IgG/IgA detection assays had an overall sensitivity of 54.4%/37.4%, 38.5%/26.8% and 52.9%/28.6% in plasma, urine and saliva specimens, respectively. IgM were detected in 38.1% and 36% of the plasma and saliva samples but never in urine.
Conclusions: Although the performances of the different diagnostic methods were not as good in saliva and urine as in plasma specimens, the results obtained by qRT-PCR and by anti-DENV antibody ELISA could well justify the use of these two body fluids to detect dengue infection in situations when the collection of blood specimens is not possible.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: PB is employed by GlaxoSmithKline. This does not alter our adherence to all PLOS NTDs policies on sharing data and materials.
Figures



Similar articles
-
Approach to non-invasive sampling in dengue diagnostics: exploring virus and NS1 antigen detection in saliva and urine of travelers with dengue.J Clin Virol. 2014 Nov;61(3):353-8. doi: 10.1016/j.jcv.2014.08.021. Epub 2014 Sep 1. J Clin Virol. 2014. PMID: 25242312
-
Evaluation of the performances of six commercial kits designed for dengue NS1 and anti-dengue IgM, IgG and IgA detection in urine and saliva clinical specimens.BMC Infect Dis. 2016 May 16;16:201. doi: 10.1186/s12879-016-1551-x. BMC Infect Dis. 2016. PMID: 27184801 Free PMC article.
-
Performance of Dengue Diagnostic Tests in a Single-Specimen Diagnostic Algorithm.J Infect Dis. 2016 Sep 15;214(6):836-44. doi: 10.1093/infdis/jiw103. Epub 2016 Mar 16. J Infect Dis. 2016. PMID: 26984143
-
Dengue: A review of laboratory diagnostics in the vaccine age.J Med Microbiol. 2024 May;73(5). doi: 10.1099/jmm.0.001833. J Med Microbiol. 2024. PMID: 38722305 Review.
-
Diagnostic approaches for dengue infection.Expert Rev Mol Diagn. 2023 Jul-Dec;23(8):643-651. doi: 10.1080/14737159.2023.2234815. Epub 2023 Jul 13. Expert Rev Mol Diagn. 2023. PMID: 37417532 Review.
Cited by
-
Temporal patterns of functional anti-dengue antibodies in dengue infected individuals with different disease outcome or infection history.Sci Rep. 2022 Oct 25;12(1):17863. doi: 10.1038/s41598-022-21722-2. Sci Rep. 2022. PMID: 36284116 Free PMC article.
-
Novel impacts of saliva with regard to oral health.J Prosthet Dent. 2022 Mar;127(3):383-391. doi: 10.1016/j.prosdent.2021.05.009. Epub 2021 Jun 16. J Prosthet Dent. 2022. PMID: 34140141 Free PMC article. Review.
-
Direct Viral RNA Detection of SARS-CoV-2 and DENV in Inactivated Samples by Real-Time RT-qPCR: Implications for Diagnosis in Resource Limited Settings with Flavivirus Co-Circulation.Pathogens. 2021 Nov 29;10(12):1558. doi: 10.3390/pathogens10121558. Pathogens. 2021. PMID: 34959513 Free PMC article.
-
Rift Valley Fever Virus and Yellow Fever Virus in Urine: A Potential Source of Infection.Virol Sin. 2019 Jun;34(3):342-345. doi: 10.1007/s12250-019-00096-2. Epub 2019 Mar 19. Virol Sin. 2019. PMID: 30888606 Free PMC article. No abstract available.
-
Yellow Fever Virus RNA in Urine and Semen of Convalescent Patient, Brazil.Emerg Infect Dis. 2018 Jan;24(1):176-8. doi: 10.3201/eid2401.171310. Epub 2018 Jan 17. Emerg Infect Dis. 2018. PMID: 29058663 Free PMC article.
References
-
- Special Programme for Research and Training in Tropical Diseases, World Health Organization Dengue: guidelines for diagnosis, treatment, prevention, and control. New ed. Geneva: TDR : World Health Organization; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

