Preclinical evaluation of injectable reduced hydroxocobalamin as an antidote to acute carbon monoxide poisoning
- PMID: 26406423
- PMCID: PMC4602171
- DOI: 10.1097/TA.0000000000000740
Preclinical evaluation of injectable reduced hydroxocobalamin as an antidote to acute carbon monoxide poisoning
Abstract
Background: Current management of acute inhalational carbon monoxide (CO) toxicity includes hyperbaric or normobaric O2 therapy. However, efficacy has not been established. The purpose of this study was to establish therapeutic proof of concept for a novel injectable antidote consisting of the combination of hydroxocobalamin and ascorbic acid into a reduced form (B12r) as demonstrated by clinically significant increase (>500 ppm) in CO2 production, reduced carboxyhemoglobin (COHgb) half-life (COHgb t1/2), and increased cerebral O2 delivery and attenuation of CO-induced microglial damage in a preclinical rodent model of CO toxicity.
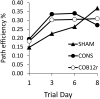
Methods: B12r-mediated conversion of CO to CO2 and COHgb t1/2 in human blood were measured by gas analysis and Raman resonance spectroscopy. Rats were exposed to either air or CO and then injected with saline or B12r. Cognitive assessment was tested in a Morris water maze. Brain oxygenation was measured with Licox. Brain histology was assessed by fluorescent antibody markers and cell counts.
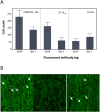
Results: B12r resulted in significant CO2 production (1,170 ppm), compared with controls. COHgb t1/2 was reduced from 33 minutes (normal saline) to 17.5 (p < 0.001). In rat models, severe CO-induced brain hypoxia (PbtO2, 18 mm Hg) was followed by significant reduction in τ25 to 12 minutes for B12r rats versus 40 minutes for normal saline-treated rats (p < 0.0001). There was major attenuation of CO-induced microglial damage, although cognitive performance differences were minimal.
Conclusion: Our preclinical data suggest that the novel synergism of hydroxocobalamin with ascorbic acid has the potential to extract CO through conversion to CO2, independently of high-flow or high-pressure O2. This resulted in a clinically significant off-gassing of CO2 at levels five to eight times greater than those of controls, a clinically significant reduction in COHgb half-life, and evidence of increased brain oxygenation and amelioration of myoglial damage in rat models. Reduced hydroxocobalamin has major potential as an injectable antidote for CO toxicity.
Conflict of interest statement
Figures



References
-
- Mowry JB, Spyker DA, Cantilena LR, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol Phila PA. 2013;51:949–1229. - PubMed
-
- Buckley NA, Juurlink DN. Carbon monoxide treatment guidelines must acknowledge the limitations of the existing evidence. Am J Respir Crit Care Med. 2013;187:390. - PubMed
-
- Schrauzer GN, Lee LP. The reduction of vitamin B12a by carbon monoxide. Arch Biochem Biophy. 1970;138:16–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

