Expression of the Receptor Tyrosine Kinase EphB2 on Dendritic Cells Is Modulated by Toll-Like Receptor Ligation but Is Not Required for T Cell Activation
- PMID: 26407069
- PMCID: PMC4583388
- DOI: 10.1371/journal.pone.0138835
Expression of the Receptor Tyrosine Kinase EphB2 on Dendritic Cells Is Modulated by Toll-Like Receptor Ligation but Is Not Required for T Cell Activation
Abstract
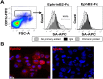
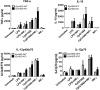
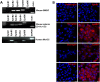
The Eph receptor tyrosine kinases interact with their ephrin ligands on adjacent cells to facilitate contact-dependent cell communication. Ephrin B ligands are expressed on T cells and have been suggested to act as co-stimulatory molecules during T cell activation. There are no detailed reports of the expression and modulation of EphB receptors on dendritic cells, the main antigen presenting cells that interact with T cells. Here we show that mouse splenic dendritic cells (DC) and bone-marrow derived DCs (BMDC) express EphB2, a member of the EphB family. EphB2 expression is modulated by ligation of TLR4 and TLR9 and also by interaction with ephrin B ligands. Co-localization of EphB2 with MHC-II is also consistent with a potential role in T cell activation. However, BMDCs derived from EphB2 deficient mice were able to present antigen in the context of MHC-II and produce T cell activating cytokines to the same extent as intact DCs. Collectively our data suggest that EphB2 may contribute to DC responses, but that EphB2 is not required for T cell activation. This result may have arisen because DCs express other members of the EphB receptor family, EphB3, EphB4 and EphB6, all of which can interact with ephrin B ligands, or because EphB2 may be playing a role in another aspect of DC biology such as migration.
Conflict of interest statement
Figures




References
-
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 238(4834):1717–1720. . - PubMed
-
- Aasheim H-C, Delabie J, Finne EF. Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood. 2005; 105(7):2869–2876. . - PubMed
-
- de Saint-Vis B, Bouchet C, Gautier G, Valladeau J, Caux C, Garrone P. Human dendritic cells express neuronal Eph receptor tyrosine kinases: role of EphA2 in regulating adhesion to fibronectin. Blood. 2003; 102(13):4431–4440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

