Natalizumab in the pediatric MS population: results of the Italian registry
- PMID: 26407848
- PMCID: PMC4583752
- DOI: 10.1186/s12883-015-0433-y
Natalizumab in the pediatric MS population: results of the Italian registry
Abstract
Background: Natalizumab is a promising option for pediatric multiple sclerosis (MS) patients with active evolution and a poor response to Interferon-beta or Glatiramer Acetate. However, no data are available in large cohorts of patients and after a long-term follow up. Our study was planned to shed lights on this topic.
Methods: A registry was established in 2007 in Italy to collect MS cases treated with Natalizumab (NA) before 18 years of age.
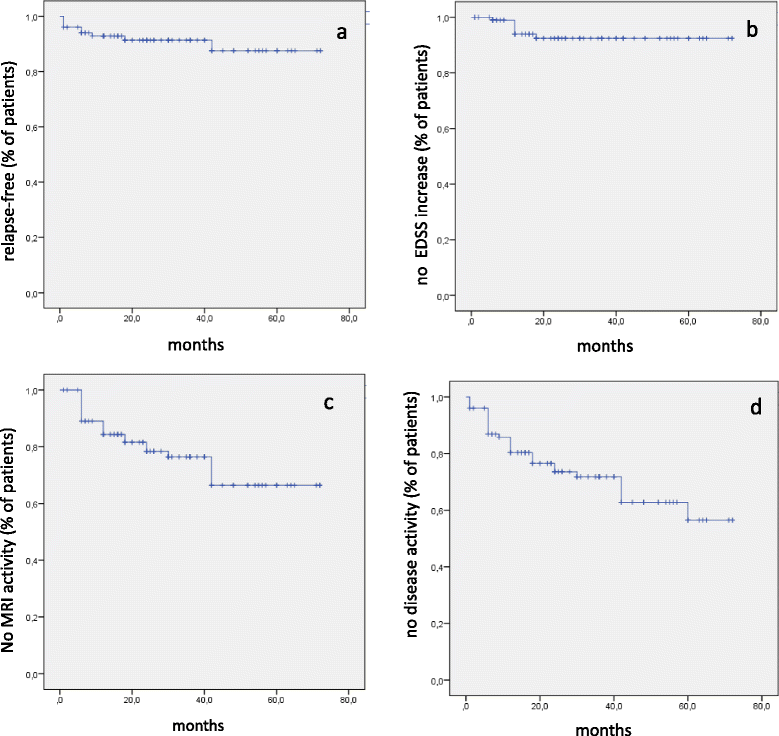
Results: 101 patients were included (69 females), mean age of MS onset 12.9 ± 2.7 years, mean age at NA initiation 14.7 ± 2.4 years. Mean treatment duration was 34.2 ± 18.3 months. During NA treatment, a total of 15 relapses were recorded in 9 patients, annualized relapse rate was 2.3 ± 1.0 in the year prior to NA and decreased to 0.1 ± 0.3 (p < 0.001) at last NA infusion. Mean Expanded Disability Status Scale (EDSS) decreased from 2.6 ± 1.3 at initiation of NA to 1.8 ± 1.2 at the time of last visit (p < 0.001). At brain MRI, new T2 or Gd enhancing lesions were observed in 10/91 patients after 6 months, 6/87 after 12 months, 2/61 after 18 months, 2/68 after 24 months, 3/62 after 30 months, and 5/43 at longer follow up. At the time of last observation, 58% of patients were free from clinical (relapses/increased EDSS) and/or MRI activity (new T2 or gadolinium-enhancing lesions). No relevant adverse events were recorded.
Discussion: NA was safe, well tolerated and very efficacious in the large majority of patients. Our data support the use of this medication in subjects with pediatric MS and an aggressive course.
Conclusions: A relevant reduction of relapse rate and EDSS was observed during NA treatment, compared to pre-treatment period. No evidence of disease activity (NEDA) occurred in 58% of cases.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical