Bioinformatic characterization of glycyl radical enzyme-associated bacterial microcompartments
- PMID: 26407889
- PMCID: PMC4644659
- DOI: 10.1128/AEM.02587-15
Bioinformatic characterization of glycyl radical enzyme-associated bacterial microcompartments
Abstract
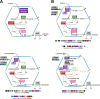
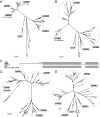
Bacterial microcompartments (BMCs) are proteinaceous organelles encapsulating enzymes that catalyze sequential reactions of metabolic pathways. BMCs are phylogenetically widespread; however, only a few BMCs have been experimentally characterized. Among them are the carboxysomes and the propanediol- and ethanolamine-utilizing microcompartments, which play diverse metabolic and ecological roles. The substrate of a BMC is defined by its signature enzyme. In catabolic BMCs, this enzyme typically generates an aldehyde. Recently, it was shown that the most prevalent signature enzymes encoded by BMC loci are glycyl radical enzymes, yet little is known about the function of these BMCs. Here we characterize the glycyl radical enzyme-associated microcompartment (GRM) loci using a combination of bioinformatic analyses and active-site and structural modeling to show that the GRMs comprise five subtypes. We predict distinct functions for the GRMs, including the degradation of choline, propanediol, and fuculose phosphate. This is the first family of BMCs for which identification of the signature enzyme is insufficient for predicting function. The distinct GRM functions are also reflected in differences in shell composition and apparently different assembly pathways. The GRMs are the counterparts of the vitamin B12-dependent propanediol- and ethanolamine-utilizing BMCs, which are frequently associated with virulence. This study provides a comprehensive foundation for experimental investigations of the diverse roles of GRMs. Understanding this plasticity of function within a single BMC family, including characterization of differences in permeability and assembly, can inform approaches to BMC bioengineering and the design of therapeutics.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

