Positive Mode LC-MS/MS Analysis of Chondroitin Sulfate Modified Glycopeptides Derived from Light and Heavy Chains of The Human Inter-α-Trypsin Inhibitor Complex
- PMID: 26407992
- PMCID: PMC4762621
- DOI: 10.1074/mcp.M115.051136
Positive Mode LC-MS/MS Analysis of Chondroitin Sulfate Modified Glycopeptides Derived from Light and Heavy Chains of The Human Inter-α-Trypsin Inhibitor Complex
Abstract
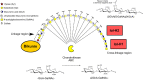
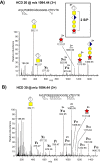
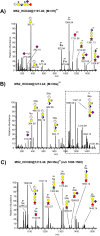
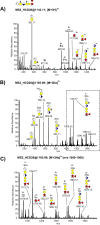
The inter-α-trypsin inhibitor complex is a macromolecular arrangement of structurally related heavy chain proteins covalently cross-linked to the chondroitin sulfate (CS) chain of the proteoglycan bikunin. The inter-α-trypsin inhibitor complex is abundant in plasma and associated with inflammation, kidney diseases, cancer and diabetes. Bikunin is modified at Ser-10 by a single low-sulfated CS chain of 23-55 monosaccharides with 4-9 sulfate groups. The innermost four monosaccharides (GlcAβ3Galβ3Galβ4Xylβ-O-) compose the linkage region, believed to be uniform with a 4-O-sulfation to the outer Gal. The cross-linkage region of the bikunin CS chain is located in the nonsulfated nonreducing end, (GalNAcβ4GlcAβ3)(n), to which heavy chains (H1-H3) may be bound in GalNAc to Asp ester linkages. In this study we employed a glycoproteomics protocol to enrich and analyze light and heavy chain linkage and cross-linkage region CS glycopeptides derived from the IαI complex of human plasma, urine and cerebrospinal fluid samples. The samples were trypsinized, enriched by strong anion exchange chromatography, partially depolymerized with chondroitinase ABC and analyzed by LC-MS/MS using higher-energy collisional dissociation. The analyses demonstrated that the CS linkage region of bikunin is highly heterogeneous. In addition to sulfation of the Gal residue, Xyl phosphorylation was observed although exclusively in urinary samples. We also identified novel Neu5Ac and Fuc modifications of the linkage region as well as the presence of mono- and disialylated core 1 O-linked glycans on Thr-17. Heavy chains H1 and H2 were identified cross-linked to GalNAc residues one or two GlcA residues apart and H1 was found linked to either the terminal or subterminal GalNAc residues. The fragmentation behavior of CS glycopeptides under variable higher-energy collisional dissociation conditions displays an energy dependence that may be used to obtain complementary structural details. Finally, we show that the analysis of sodium adducts provides confirmatory information about the positions of glycan substituents.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Thaysen-Andersen M., and Packer N. H. (2014) Advances in LC-MS/MS-based glycoproteomics: Getting closer to system-wide site-specific mapping of the N- and O-glycoproteome. Biochim Biophys Acta-Proteins Proteom 1844, 1437–1452 - PubMed
-
- Esko J. D., Kimata K., and Lindahl U. (2009) Proteoglycans and Sulfated Glycosaminoglycans. in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., and Etzler M. E. eds.), 2nd Ed., Cold Spring Harbor (NY) pp 229–248 - PubMed
-
- Pickford C. E., Holley R. J., Rushton G., Stavridis M. P., Ward C. M., and Merry C. L. (2011) Specific glycosaminoglycans modulate neural specification of mouse embryonic stem cells. Stem Cells 29, 629–640 - PubMed
-
- Chan C. K., Rolle M. W., Potter-Perigo S., Braun K. R., Van Biber B. P., Laflamme M. A., Murry C. E., and Wight T. N. (2010) Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan. J. Cell. Biochem. 111, 585–596 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

