Chronic stress and peripheral pain: Evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathways
- PMID: 26408049
- PMCID: PMC4644500
- DOI: 10.1016/j.expneurol.2015.09.013
Chronic stress and peripheral pain: Evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathways
Erratum in
-
Corrigendum to "Chronic stress and peripheral pain: Evidence for distinct, region-specific changes in visceral and somatosensory pain regulatory pathways" [Exp Neurol. 2015 Nov.; 273: 301-11].Exp Neurol. 2016 May;279:290. doi: 10.1016/j.expneurol.2016.03.003. Epub 2016 Mar 8. Exp Neurol. 2016. PMID: 27085462 No abstract available.
Abstract
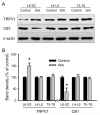
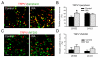
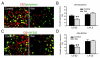
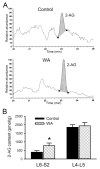
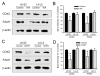
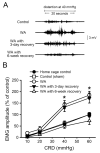
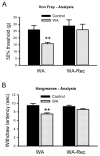
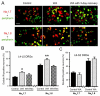
Chronic stress alters the hypothalamic-pituitary-adrenal (HPA) axis and enhances visceral and somatosensory pain perception. It is unresolved whether chronic stress has distinct effects on visceral and somatosensory pain regulatory pathways. Previous studies reported that stress-induced visceral hyperalgesia is associated with reciprocal alterations of endovanilloid and endocannabinoid pain pathways in DRG neurons innervating the pelvic viscera. In this study, we compared somatosensory and visceral hyperalgesia with respect to differential responses of peripheral pain regulatory pathways in a rat model of chronic, intermittent stress. We found that chronic stress induced reciprocal changes in the endocannabinoid 2-AG (increased) and endocannabinoid degradation enzymes COX-2 and FAAH (decreased), associated with down-regulation of CB1 and up-regulation of TRPV1 receptors in L6-S2 DRG but not L4-L5 DRG neurons. In contrast, sodium channels Nav1.7 and Nav1.8 were up-regulated in L4-L5 but not L6-S2 DRGs in stressed rats, which was reproduced in control DRGs treated with corticosterone in vitro. The reciprocal changes of CB1, TRPV1 and sodium channels were cell-specific and observed in the sub-population of nociceptive neurons. Behavioral assessment showed that visceral hyperalgesia persisted, whereas somatosensory hyperalgesia and enhanced expression of Nav1.7 and Nav1.8 sodium channels in L4-L5 DRGs normalized 3 days after completion of the stress phase. These data indicate that chronic stress induces visceral and somatosensory hyperalgesia that involves differential changes in endovanilloid and endocannabinoid pathways, and sodium channels in DRGs innervating the pelvic viscera and lower extremities. These results suggest that chronic stress-induced visceral and lower extremity somatosensory hyperalgesia can be treated selectively at different levels of the spinal cord.
Keywords: CB1; Chronic stress; Endocannabinoid; Pain; Sodium channel; Somatosensory hyperalgesia; Trpv1; Visceral hyperalgesia.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system.Gastroenterology. 2015 Jan;148(1):148-157.e7. doi: 10.1053/j.gastro.2014.09.032. Epub 2014 Sep 28. Gastroenterology. 2015. PMID: 25263804 Free PMC article.
-
Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat.Gut. 2009 Feb;58(2):202-10. doi: 10.1136/gut.2008.157594. Epub 2008 Oct 20. Gut. 2009. PMID: 18936104 Free PMC article.
-
Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat.Gastroenterology. 2011 Feb;140(2):627-637.e4. doi: 10.1053/j.gastro.2010.11.003. Epub 2010 Nov 9. Gastroenterology. 2011. PMID: 21070780 Free PMC article.
-
Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system.Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3286-99. doi: 10.1098/rstb.2011.0392. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108547 Free PMC article. Review.
-
Interplay between endocannabinoid and endovanilloid mechanisms in fear conditioning.Acta Neuropsychiatr. 2024 Oct;36(5):255-264. doi: 10.1017/neu.2023.54. Epub 2023 Nov 20. Acta Neuropsychiatr. 2024. PMID: 37982167 Review.
Cited by
-
Nociceptor Overexpression of NaV1.7 Contributes to Chronic Muscle Pain Induced by Early-Life Stress.J Pain. 2021 Jul;22(7):806-816. doi: 10.1016/j.jpain.2021.02.003. Epub 2021 Feb 24. J Pain. 2021. PMID: 33636374 Free PMC article.
-
Ovariectomy-Induced Mitochondrial Oxidative Stress, Apoptosis, and Calcium Ion Influx Through TRPA1, TRPM2, and TRPV1 Are Prevented by 17β-Estradiol, Tamoxifen, and Raloxifene in the Hippocampus and Dorsal Root Ganglion of Rats.Mol Neurobiol. 2017 Dec;54(10):7620-7638. doi: 10.1007/s12035-016-0232-5. Epub 2016 Nov 10. Mol Neurobiol. 2017. PMID: 27832523
-
The Role of Epigenomic Regulatory Pathways in the Gut-Brain Axis and Visceral Hyperalgesia.Cell Mol Neurobiol. 2022 Mar;42(2):361-376. doi: 10.1007/s10571-021-01108-0. Epub 2021 May 31. Cell Mol Neurobiol. 2022. PMID: 34057682 Free PMC article. Review.
-
Excitation-Inhibition Imbalance Leads to Alteration of Neuronal Coherence and Neurovascular Coupling under Acute Stress.J Neurosci. 2020 Nov 18;40(47):9148-9162. doi: 10.1523/JNEUROSCI.1553-20.2020. Epub 2020 Oct 21. J Neurosci. 2020. PMID: 33087471 Free PMC article.
-
Neurovascular Coupling under Chronic Stress Is Modified by Altered GABAergic Interneuron Activity.J Neurosci. 2019 Dec 11;39(50):10081-10095. doi: 10.1523/JNEUROSCI.1357-19.2019. Epub 2019 Oct 31. J Neurosci. 2019. PMID: 31672788 Free PMC article.
References
-
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. - PubMed
-
- Bagal SK, Chapman ML, Marron BE, Prime R, Storer RI, Swain NA. Recent progress in sodium channel modulators for pain. Bioorg Med Chem Lett. 2014;24:3690–3699. - PubMed
-
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di M,V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. - PMC - PubMed
-
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

