Truncated Amyloid-β(11-40/42) from Alzheimer Disease Binds Cu2+ with a Femtomolar Affinity and Influences Fiber Assembly
- PMID: 26408196
- PMCID: PMC4646025
- DOI: 10.1074/jbc.M115.684084
Truncated Amyloid-β(11-40/42) from Alzheimer Disease Binds Cu2+ with a Femtomolar Affinity and Influences Fiber Assembly
Abstract
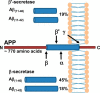
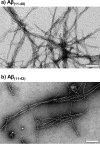
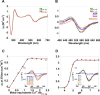
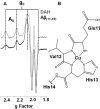
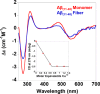
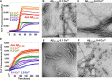
Alzheimer disease coincides with the formation of extracellular amyloid plaques composed of the amyloid-β (Aβ) peptide. Aβ is typically 40 residues long (Aβ(1-40)) but can have variable C and N termini. Naturally occurring N-terminally truncated Aβ(11-40/42) is found in the cerebrospinal fluid and has a similar abundance to Aβ(1-42), constituting one-fifth of the plaque load. Based on its specific N-terminal sequence we hypothesized that truncated Aβ(11-40/42) would have an elevated affinity for Cu(2+). Various spectroscopic techniques, complemented with transmission electron microscopy, were used to determine the properties of the Cu(2+)-Aβ(11-40/42) interaction and how Cu(2+) influences amyloid fiber formation. We show that Cu(2+)-Aβ(11-40) forms a tetragonal complex with a 34 ± 5 fm dissociation constant at pH 7.4. This affinity is 3 orders of magnitude tighter than Cu(2+) binding to Aβ(1-40/42) and more than an order of magnitude tighter than that of serum albumin, the extracellular Cu(2+) transport protein. Furthermore, Aβ(11-40/42) forms fibers twice as fast as Aβ(1-40) with a very different morphology, forming bundles of very short amyloid rods. Substoichiometric Cu(2+) drastically perturbs Aβ(11-40/42) assembly, stabilizing much longer fibers. The very tight fm affinity of Cu(2+) for Aβ(11-40/42) explains the high levels of Cu(2+) observed in Alzheimer disease plaques.
Keywords: Alzheimer disease; affinity; albumin; amyloid; amyloid-β (AB); circular dichroism (CD); coordination; copper; fiber kinetics; metal ion-protein interaction.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Prince M., Jackson J., Ferri C. P., Sousa R., Albanese E., Ribeiro W. S., and Honyashiki M. (2009) Alzheimer's disease international world Alzheimer report. In International AsD (ed), pp. 1–96, Alzheimer's Disease International, London
-
- Hardy J. A., and Higgins G. A. (1992) Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185 - PubMed
-
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., and Müller-Hill B. (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325, 733–736 - PubMed
-
- Prelli F., Castaño E., Glenner G. G., and Frangione B. (1988) Differences between vascular and plaque core amyloid in Alzheimer's disease. J. Neurochem. 51, 648–651 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

