Interleukin 10 (IL-10)-mediated Immunosuppression: MARCH-I INDUCTION REGULATES ANTIGEN PRESENTATION BY MACROPHAGES BUT NOT DENDRITIC CELLS
- PMID: 26408197
- PMCID: PMC4646393
- DOI: 10.1074/jbc.M115.682708
Interleukin 10 (IL-10)-mediated Immunosuppression: MARCH-I INDUCTION REGULATES ANTIGEN PRESENTATION BY MACROPHAGES BUT NOT DENDRITIC CELLS
Abstract
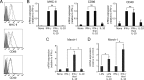
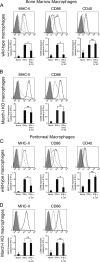
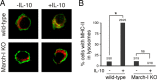
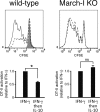
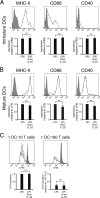
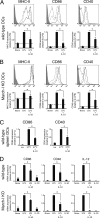
Efficient immune responses require regulated antigen presentation to CD4 T cells. IL-10 inhibits the ability of dendritic cells (DCs) and macrophages to stimulate antigen-specific CD4 T cells; however, the mechanisms by which IL-10 suppresses antigen presentation remain poorly understood. We now report that IL-10 stimulates expression of the E3 ubiquitin ligase March-I in activated macrophages, thereby down-regulating MHC-II, CD86, and antigen presentation to CD4 T cells. By contrast, IL-10 does not stimulate March-I expression in DCs, does not suppress MHC-II or CD86 expression on either resting or activated DCs, and does not affect antigen presentation by activated DCs. IL-10 does, however, inhibit the process of DC activation itself, thereby reducing the efficiency of antigen presentation in a March-I-independent manner. Thus, IL-10 suppression of antigen presenting cell function in macrophages is March-I-dependent, whereas in DCs, suppression is March- I-independent.
Keywords: antigen presentation; dendritic cell; immunosuppression; interleukin; macrophage; major histocompatibility complex (MHC); ubiquitin ligase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Itano A. A., and Jenkins M. K. (2003) Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4, 733–739 - PubMed
-
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., and Palucka K. (2000) Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 - PubMed
-
- Kawai T., and Akira S. (2006) TLR signaling. Cell Death Differ. 13, 816–825 - PubMed
-
- Schroder K., Hertzog P. J., Ravasi T., and Hume D. A. (2004) Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

