Membrane Potential Controls the Efficacy of Catecholamine-induced β1-Adrenoceptor Activity
- PMID: 26408198
- PMCID: PMC4646385
- DOI: 10.1074/jbc.M115.665000
Membrane Potential Controls the Efficacy of Catecholamine-induced β1-Adrenoceptor Activity
Abstract
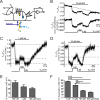
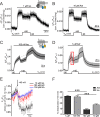
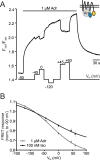
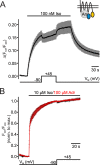
G protein-coupled receptors (GPCRs) are membrane-located proteins and, therefore, are exposed to changes in membrane potential (V(M)) in excitable tissues. These changes have been shown to alter receptor activation of certain Gi-and Gq-coupled GPCRs. By means of a combination of whole-cell patch-clamp and Förster resonance energy transfer (FRET) in single cells, we demonstrate that the activation of the Gs-coupled β1-adrenoreceptor (β1-AR) by the catecholamines isoprenaline (Iso) and adrenaline (Adr) is regulated by V(M). This voltage-dependence is also transmitted to G protein and arrestin 3 signaling. Voltage-dependence of β2-AR activation, however, was weak compared with β1-AR voltage-dependence. Drug efficacy is a major target of β1-AR voltage-dependence as depolarization attenuated receptor activation, even under saturating concentrations of agonists, with significantly faster kinetics than the deactivation upon agonist withdrawal. Also the efficacy of the endogenous full agonist adrenaline was reduced by depolarization. This is a unique finding since reports of natural full agonists at other voltage-dependent GPCRs only show alterations in affinity during depolarization. Based on a Boltzmann function fit to the relationship of V(M) and receptor-arrestin 3 interaction we determined the voltage-dependence with highest sensitivity in the physiological range of membrane potential. Our data suggest that under physiological conditions voltage regulates the activity of agonist-occupied β1-adrenoceptors on a very fast time scale.
Keywords: G protein; G protein-coupled receptor (GPCR); adrenergic receptor; arrestin; fluorescence resonance energy transfer (FRET); kinetics; voltage-dependence.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Ben-Chaim Y., Tour O., Dascal N., Parnas I., and Parnas H. (2003) The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J. Biol. Chem. 278, 22482–22491 - PubMed
-
- Ohana L., Barchad O., Parnas I., and Parnas H. (2006) The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J. Biol. Chem. 281, 24204–24215 - PubMed
-
- Martinez-Pinna J., Gurung I. S., Vial C., Leon C., Gachet C., Evans R. J., and Mahaut-Smith M. P. (2005) Direct voltage control of signaling via P2Y1 and other Gαq-coupled receptors. J. Biol. Chem. 280, 1490–1498 - PubMed
-
- Sahlholm K., Nilsson J., Marcellino D., Fuxe K., and Arhem P. (2008) Voltage-dependence of the human dopamine D2 receptor. Synapse 62, 476–480 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

