Sulodexide for the Prevention of Recurrent Venous Thromboembolism: The Sulodexide in Secondary Prevention of Recurrent Deep Vein Thrombosis (SURVET) Study: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 26408273
- PMCID: PMC4643750
- DOI: 10.1161/CIRCULATIONAHA.115.016930
Sulodexide for the Prevention of Recurrent Venous Thromboembolism: The Sulodexide in Secondary Prevention of Recurrent Deep Vein Thrombosis (SURVET) Study: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Background: Patients with a first episode of unprovoked venous thromboembolism have a high risk of recurrence after discontinuation of anticoagulant therapy. Extending anticoagulation reduces the risk of recurrence but is associated with increased bleeding. Sulodexide, a glycosaminoglycan, exerts antithrombotic and profibrinolytic actions with a low bleeding risk when administered orally, but its benefit for preventing recurrent venous thromboembolism is not well known.
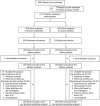
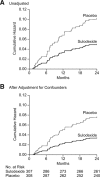
Methods and results: In this multicenter, double-blind study, 615 patients with first-ever unprovoked venous thromboembolism who had completed 3 to 12 months of oral anticoagulant treatment were randomly assigned to sulodexide 500 lipasemic units twice daily or placebo for 2 years, in addition to elastic stockings. The primary efficacy outcome was recurrence of venous thromboembolism. Major or clinically relevant bleeding was the primary safety outcome. Venous thromboembolism recurred in 15 of the 307 patients who received sulodexide and in 30 of the 308 patients who received placebo (hazard ratio, 0.49; 95% confidence interval [CI], 0.27-0.92; P=0.02). The analysis in which lost to follow-up was assigned to failure yielded a risk ratio among treated versus control subjects of 0.54 (95% confidence interval, 0.35-0.85; P=0.009). No major bleeding episodes occurred; 2 patients in each treatment group had a clinically relevant bleeding episode. Adverse events were similar in the 2 groups.
Conclusion: Sulodexide given after discontinuation of anticoagulant treatment reduced the risk of recurrence in patients with unprovoked venous thromboembolism, with no apparent increase of bleeding risk.
Clinical trial registration: URL: https://www.clinicaltrialsregister.eu/. Identifier: EudraCT number 2009-016923-77.
Keywords: glycosaminoglycans; randomized controlled trial; recurrence; venous thromboembolism.
© 2015 The Authors.
Figures
Comment in
-
Secondary Prevention of Venous Thromboembolism: One Regimen May Not Fit All.Circulation. 2015 Nov 17;132(20):1856-9. doi: 10.1161/CIRCULATIONAHA.115.019310. Epub 2015 Sep 25. Circulation. 2015. PMID: 26408272 No abstract available.
-
Anticoagulation therapy: Sulodexide reduces the risk of recurrent venous thromboembolism.Nat Rev Cardiol. 2015 Dec;12(12):682. doi: 10.1038/nrcardio.2015.163. Epub 2015 Oct 13. Nat Rev Cardiol. 2015. PMID: 26461966 No abstract available.
References
-
- Schafer AI. Venous thrombosis as a chronic disease. N Engl J Med. 1999;340:955–956. doi: 10.1056/NEJM199903253401209. - PubMed
-
- Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. - PubMed
-
- Schulman S, Rhedin AS, Lindmarker P, Carlsson A, Lärfars G, Nicol P, Loogna E, Svensson E, Ljungberg B, Walter H. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism: Duration of Anticoagulation Trial Study Group. N Engl J Med. 1995;332:1661–1665. doi: 10.1056/NEJM199506223322501. - PubMed
-
- Agnelli G, Prandoni P, Santamaria MG, Bagatella P, Iorio A, Bazzan M, Moia M, Guazzaloca G, Bertoldi A, Tomasi C, Scannapieco G, Ageno W Warfarin Optimal Duration Italian Trial Investigators. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis: Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med. 2001;345:165–169. doi: 10.1056/NEJM200107193450302. - PubMed
-
- Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, Turpie AG, Green D, Ginsberg JS, Wells P, MacKinnon B, Julian JA. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–907. doi: 10.1056/NEJM199903253401201. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

