Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease
- PMID: 26408528
- PMCID: PMC4630566
- DOI: 10.1124/pr.114.010397
Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease
Abstract
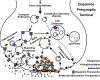
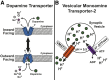
Dopamine (DA) plays a well recognized role in a variety of physiologic functions such as movement, cognition, mood, and reward. Consequently, many human disorders are due, in part, to dysfunctional dopaminergic systems, including Parkinson's disease, attention deficit hyperactivity disorder, and substance abuse. Drugs that modify the DA system are clinically effective in treating symptoms of these diseases or are involved in their manifestation, implicating DA in their etiology. DA signaling and distribution are primarily modulated by the DA transporter (DAT) and by vesicular monoamine transporter (VMAT)-2, which transport DA into presynaptic terminals and synaptic vesicles, respectively. These transporters are regulated by complex processes such as phosphorylation, protein-protein interactions, and changes in intracellular localization. This review provides an overview of 1) the current understanding of DAT and VMAT2 neurobiology, including discussion of studies ranging from those conducted in vitro to those involving human subjects; 2) the role of these transporters in disease and how these transporters are affected by disease; and 3) and how selected drugs alter the function and expression of these transporters. Understanding the regulatory processes and the pathologic consequences of DAT and VMAT2 dysfunction underlies the evolution of therapeutic development for the treatment of DA-related disorders.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Adkins EM, Samuvel DJ, Fog JU, Eriksen J, Jayanthi LD, Vaegter CB, Ramamoorthy S, Gether U. (2007) Membrane mobility and microdomain association of the dopamine transporter studied with fluorescence correlation spectroscopy and fluorescence recovery after photobleaching. Biochemistry 46:10484–10497. - PubMed
-
- Afonso-Oramas D, Cruz-Muros I, Alvarez de la Rosa D, Abreu P, Giráldez T, Castro-Hernández J, Salas-Hernández J, Lanciego JL, Rodríguez M, González-Hernández T. (2009) Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol Dis 36:494–508. - PubMed
-
- Anker JJ, Carroll ME. (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96. - PubMed
-
- Assmann BE, Robinson RO, Surtees RA, Bräutigam C, Heales SJ, Wevers RA, Zschocke J, Hyland K, Sharma R, Hoffmann GF. (2004) Infantile Parkinsonism-dystonia and elevated dopamine metabolites in CSF. Neurology 62:1872–1874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

