Perspectives on Advances in Tuberculosis Diagnostics, Drugs, and Vaccines
- PMID: 26409271
- PMCID: PMC4583570
- DOI: 10.1093/cid/civ609
Perspectives on Advances in Tuberculosis Diagnostics, Drugs, and Vaccines
Abstract
Despite concerted efforts over the past 2 decades at developing new diagnostics, drugs, and vaccines with expanding pipelines, tuberculosis remains a global emergency. Several novel diagnostic technologies show promise of better point-of-care rapid tests for tuberculosis including nucleic acid-based amplification tests, imaging, and breath analysis of volatile organic compounds. Advances in new and repurposed drugs for use in multidrug-resistant (MDR) or extensively drug-resistant (XDR) tuberculosis have focused on development of several new drug regimens and their evaluation in clinical trials and now influence World Health Organization guidelines. Since the failure of the MVA85A vaccine 2 years ago, there have been no new tuberculosis vaccine candidates entering clinical testing. The current status quo of the lengthy treatment duration and poor treatment outcomes associated with MDR/XDR tuberculosis and with comorbidity of tuberculosis with human immunodeficiency virus and noncommunicable diseases is unacceptable. New innovations and political and funder commitment for early rapid diagnosis, shortening duration of therapy, improving treatment outcomes, and prevention are urgently required.
Keywords: diagnostics; drugs; management; tuberculosis; vaccines.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
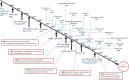
References
-
- World Health Organization. Global tuberculosis report 2014. Available at: http://www.who.int/tb/publications/global_report/en/ Accessed 24 March 2015.
-
- Bates M, Mudenda V, Shibemba A, et al. Burden of tuberculosis at post-mortem in inpatient adults at a tertiary referral centre in sub-Saharan Africa—a prospective descriptive autopsy study. Lancet Infect Dis 2015; 5:544–51. - PubMed
-
- Cox JA, Lukande RL, Lucas S, Nelson AM, Van Marck E, Colebunders R. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS Rev 2010; 12:183–94. - PubMed
-
- Bates M, Marais BJ, Zumla A. Tuberculosis comorbidity with communicable and noncommunicable diseases. Cold Spring Harb Perspect Med 2015; doi:10.1101/cshperspect.a017889. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

