Immune Cell Regulatory Pathways Unexplored as Host-Directed Therapeutic Targets for Mycobacterium tuberculosis: An Opportunity to Apply Precision Medicine Innovations to Infectious Diseases
- PMID: 26409283
- PMCID: PMC4583576
- DOI: 10.1093/cid/civ621
Immune Cell Regulatory Pathways Unexplored as Host-Directed Therapeutic Targets for Mycobacterium tuberculosis: An Opportunity to Apply Precision Medicine Innovations to Infectious Diseases
Abstract
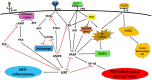
The lack of novel antimicrobial drugs in development for tuberculosis treatment has provided an impetus for the discovery of adjunctive host-directed therapies (HDTs). Several promising HDT candidates are being evaluated, but major advancement of tuberculosis HDTs will require understanding of the master or "core" cell signaling pathways that control intersecting immunologic and metabolic regulatory mechanisms, collectively described as "immunometabolism." Core regulatory pathways conserved in all eukaryotic cells include poly (ADP-ribose) polymerases (PARPs), sirtuins, AMP-activated protein kinase (AMPK), and mechanistic target of rapamycin (mTOR) signaling. Critical interactions of these signaling pathways with each other and their roles as master regulators of immunometabolic functions will be addressed, as well as how Mycobacterium tuberculosis is already known to influence various other cell signaling pathways interacting with them. Knowledge of these essential mechanisms of cell function regulation has led to breakthrough targeted treatment advances for many diseases, most prominently in oncology. Leveraging these exciting advances in precision medicine for the development of innovative next-generation HDTs may lead to entirely new paradigms for treatment and prevention of tuberculosis and other infectious diseases.
Keywords: host-directed therapy; immunometabolism; precision medicine; signaling pathways; tuberculosis.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

