Establishment and application of cross-priming isothermal amplification coupled with lateral flow dipstick (CPA-LFD) for rapid and specific detection of red-spotted grouper nervous necrosis virus
- PMID: 26409445
- PMCID: PMC4583742
- DOI: 10.1186/s12985-015-0374-5
Establishment and application of cross-priming isothermal amplification coupled with lateral flow dipstick (CPA-LFD) for rapid and specific detection of red-spotted grouper nervous necrosis virus
Abstract
Background: Red-spotted grouper nervous necrosis virus (RGNNV) is an important pathogen that causes diseases in many species of fish in marine aquaculture. The larvae and juveniles are more easily infected by RGNNV and the cumulative mortality is as high as 100 % after being infected with RGNNV. This virus imposes a serious threat to aquaculture of grouper fry. This study aimed to establish a simple, accurate and highly sensitive method for rapid detection of RGNNV on the spot.
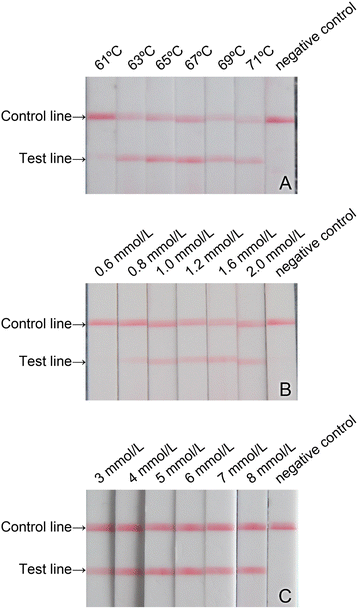
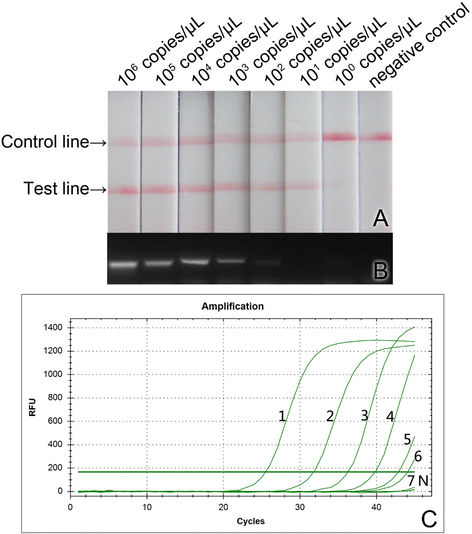
Methods: In this study, the primers specifically targeting RGNNV were designed and cross-priming isothermal amplification (CPA) system was established. The product amplified by CPA was detected through visualization with lateral flow dipstick (LFD). Three important parameters, including the amplification temperature, the concentration of dNTPs and the concentration of Mg(2+) for the CPA system, were optimized. The sensitivity and specificity of this method for RGNNV were tested and compared with those of the conventional RT-PCR and real-time quantitative RT-PCR (qRT-PCR).
Results: The optimized conditions for the CPA amplification system were determined as follows: the optimal amplification temperature, the optimized concentration of dNTPs and the concentration for Mg(2+) were 69 °C, 1.2 mmol/L and 5 mmol/L, respectively. The lowest limit of detection (LLOD) of this method for RGNNV was 10(1) copies/μL of RNA sample, which was 10 times lower than that of conventional RT-PCR and comparable to that of RT-qPCR. This method was specific for RGNNV in combination with SJNNV and had no cross-reactions with 8 types of virus and bacterial strains tested. This method was successfully applied to detect RGNNV in fish samples.
Conclusions: This study established a CPA-LFD method for detection of RGNNV. This method is simple and rapid with high sensitivity and good specificity and can be widely applied for rapid detection of this virus on the spot.
Figures



References
-
- Johnson SC, Sperker SA, Leggiadro CT, Groman DB, Griffiths SG, Ritchie RJ, Cook MD, Cusack RR. Identification and characterization of a piscine neuropathy and nodavirus from juvenile Atlantic cod from the Atlantic coast of north America. J Aquat Anim Health. 2002;14:124–133. doi: 10.1577/1548-8667(2002)014<0124:IACOAP>2.0.CO;2. - DOI
-
- Arimoto M, Mushiake K, Mizuta Y, Nakai T, Muroga K, Furusawa I. Detection of striped jack nervous necrosis virus (SJNNV) by enzyme-linked immunosorbent assay (ELISA) Fish Pathol. 1992;27:191–195. doi: 10.3147/jsfp.27.191. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

