Clinical Outcome Assessments: Conceptual Foundation-Report of the ISPOR Clinical Outcomes Assessment - Emerging Good Practices for Outcomes Research Task Force
- PMID: 26409600
- PMCID: PMC4610138
- DOI: 10.1016/j.jval.2015.08.006
Clinical Outcome Assessments: Conceptual Foundation-Report of the ISPOR Clinical Outcomes Assessment - Emerging Good Practices for Outcomes Research Task Force
Abstract
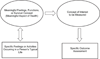
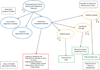
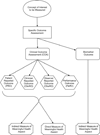
An outcome assessment, the patient assessment used in an endpoint, is the measuring instrument that provides a rating or score (categorical or continuous) that is intended to represent some aspect of the patient's health status. Outcome assessments are used to define efficacy endpoints when developing a therapy for a disease or condition. Most efficacy endpoints are based on specified clinical assessments of patients. When clinical assessments are used as clinical trial outcomes, they are called clinical outcome assessments (COAs). COAs include any assessment that may be influenced by human choices, judgment, or motivation. COAs must be well-defined and possess adequate measurement properties to demonstrate (directly or indirectly) the benefits of a treatment. In contrast, a biomarker assessment is one that is subject to little, if any, patient motivational or rater judgmental influence. This is the first of two reports by the ISPOR Clinical Outcomes Assessment - Emerging Good Practices for Outcomes Research Task Force. This report provides foundational definitions important for an understanding of COA measurement principles. The foundation provided in this report includes what it means to demonstrate a beneficial effect, how assessments of patients relate to the objective of showing a treatment's benefit, and how these assessments are used in clinical trial endpoints. In addition, this report describes intrinsic attributes of patient assessments and clinical trial factors that can affect the properties of the measurements. These factors should be considered when developing or refining assessments. These considerations will aid investigators designing trials in their choice of using an existing assessment or developing a new outcome assessment. Although the focus of this report is on the development of a new COA to define endpoints in a clinical trial, these principles may be applied more generally. A critical element in appraising or developing a COA is to describe the treatment's intended benefit as an effect on a clearly identified aspect of how a patient feels or functions. This aspect must have importance to the patient and be part of the patient's typical life. This meaningful health aspect can be measured directly or measured indirectly when it is impractical to evaluate it directly or when it is difficult to measure. For indirect measurement, a concept of interest (COI) can be identified. The COI must be related to how a patient feels or functions. Procedures are then developed to measure the COI. The relationship of these measurements with how a patient feels or functions in the intended setting and manner of use of the COA (the context of use) could then be defined. A COA has identifiable attributes or characteristics that affect the measurement properties of the COA when used in endpoints. One of these features is whether judgment can influence the measurement, and if so, whose judgment. This attribute defines four categories of COAs: patient reported outcomes, clinician reported outcomes, observer reported outcomes, and performance outcomes. A full description as well as explanation of other important COA features is included in this report. The information in this report should aid in the development, refinement, and standardization of COAs, and, ultimately, improve their measurement properties.
Keywords: clinical outcome assessment; concept of interest; context of use; treatment benefit.
Copyright © 2015 International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
ISPOR Task Force For Clinical Outcomes Assessment: Clinical Outcome Assessments: Conceptual Foundation-Report of The ISPOR Clinical Outcomes Assessment - Emerging Good Practices For Outcomes Research Task Force.Value Health. 2015 Sep;18(6):739-40. doi: 10.1016/j.jval.2015.09.2863. Value Health. 2015. PMID: 26409599 No abstract available.
References
-
- Johansson GM, Hager CK. Measurement properties of the Motor Evaluation Scale for Upper Extremity in Stroke patients (MESUPES) Disabil Rehabil. 2012;34:288–294. - PubMed
-
- Coster WJ, Haley SM, Ludlow LH, et al. Development of an applied cognition scale to measure rehabilitation outcomes. Arch Phys Med Rehabil. 2004;85:2030–2035. - PubMed
-
- Burk K, Schulz SR, Schulz JB. Monitoring progression in Friedreich ataxia (FRDA): the use of clinical scales. J Neurochem. 2013;126(Suppl. 1):118–124. - PubMed
-
- Howard K, Berry P, Petrillo J, et al. Development of the Shortness of Breath with Daily Activities questionnaire (SOBDA) Value Health. 2012;15:1042–1050. - PubMed
-
- Padua L, Evoli A, Aprile I, et al. Myasthenia gravis outcome measure: development and validation of a disease-specific self-administered questionnaire. Neurol Sci. 2012;23:59–68. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

