Buprenorphine dose induction in non-opioid-tolerant pre-release prisoners
- PMID: 26409751
- PMCID: PMC4633333
- DOI: 10.1016/j.drugalcdep.2015.09.001
Buprenorphine dose induction in non-opioid-tolerant pre-release prisoners
Abstract
Background: In a previously reported randomized controlled trial, formerly opioid-dependent prisoners were more likely to enter community drug abuse treatment when they were inducted in prison onto buprenorphine/naloxone (hereafter called buprenorphine) than when they received counseling without buprenorphine in prison (47.5% vs. 33.7%, p=0.012) (Gordon et al., 2014). In this communication we report on the results of the induction schedule and the adverse event profile seen in pre-release prisoners inducted onto buprenorphine.
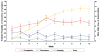
Method: This paper examines the dose induction procedure, a comparison of the proposed versus actual doses given per week, and side effects reported for 104 adult participants who were randomized to buprenorphine treatment in prison. Self-reported side effects were analyzed using generalized estimated equations to determine changes over time in side effects.
Results: Study participants were inducted onto buprenorphine at a rate faster than the induction schedule. Of the 104 (72 males, 32 females) buprenorphine recipients, 64 (37 males, 27 females) remained on medication at release from prison. Nine participants (8.6%) discontinued buprenorphine because of unpleasant opioid side effects. There were no serious adverse events reported during the in-prison phase of the study. Constipation was the most frequent symptom reported (69 percent).
Conclusion: Our findings suggest that buprenorphine administered to non-opioid-tolerant adults should be started at a lower, individualized dose than customarily used for adults actively using opioids, and that non-opioid-tolerant pre-release prisoners can be successfully inducted onto therapeutic doses prior to release.
Keywords: Buprenorphine dose; Buprenorphine induction; Opioid-dependent prisoners.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Authors Gordon, Kinlock, Jaffe, and Fitzgerald report no conflicts of interest. Drs. Vocci, Schwartz, and O’Grady have, in the past, received reimbursement for their time from Reckitt-Benckiser, one of the manufacturers of buprenorphine. Dr. Vocci has also consulted for generic buprenorphine manufacturers, and been a consultant and investigator for studies funded by Titan Pharmaceuticals and Braeburn Pharmaceuticals. All consulting fees for Drs. Vocci and Schwartz went directly to their employer, Friends Research Institute. Reckitt-Benckiser also supplied buprenorphine/naloxone tablets and film for the study. Reckitt-Benckiser had no role in the design, data collection, and analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The authors alone are responsible for the content and writing of the manuscript.
Figures
References
-
- Amass L, Bickel WK, Crean JP, Blake J, Higgins ST. Alternate-day buprenorphine dosing is preferred to daily dosing by opioid-dependent humans. Psychopharmacology. 1998;136:217–225. - PubMed
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: 1994.
-
- Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996–99. Addiction (Abingdon, England) 2003;98:185–190. - PubMed
-
- Dole VP, Robinson JW, Orraca J, Towns E, Searcy P, Caine E. Methadone treatment of randomly selected criminal addicts. N Engl J Med. 1969;280:1372–1375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

