A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma
- PMID: 26410620
- PMCID: PMC6279094
- DOI: 10.1093/annonc/mdv324
A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma
Abstract
Background: The efficacy and safety of nab-paclitaxel versus dacarbazine in patients with metastatic melanoma was evaluated in a phase III randomized, controlled trial.
Patients and methods: Chemotherapy-naïve patients with stage IV melanoma received nab-paclitaxel 150 mg/m(2) on days 1, 8, and 15 every 4 weeks or dacarbazine 1000 mg/m(2) every 3 weeks. The primary end point was progression-free survival (PFS) by independent radiologic review; the secondary end point was overall survival (OS).
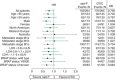
Results: A total of 529 patients were randomized to nab-paclitaxel (n = 264) or dacarbazine (n = 265). Baseline characteristics were well balanced. The majority of patients were men (66%), had an Eastern Cooperative Oncology Group status of 0 (71%), and had M1c stage disease (65%). The median PFS (primary end point) was 4.8 months with nab-paclitaxel and 2.5 months with dacarbazine [hazard ratio (HR), 0.792; 95.1% confidence interval (CI) 0.631-0.992; P = 0.044]. The median OS was 12.6 months with nab-paclitaxel and 10.5 months with dacarbazine (HR, 0.897; 95.1% CI 0.738-1.089; P = 0.271). Independently assessed overall response rate was 15% versus 11% (P = 0.239), and disease control rate (DCR) was 39% versus 27% (P = 0.004) for nab-paclitaxel versus dacarbazine, respectively. The most common grade ≥3 treatment-related adverse events were neuropathy (nab-paclitaxel, 25% versus dacarbazine, 0%; P < 0.001), and neutropenia (nab-paclitaxel, 20% versus dacarbazine, 10%; P = 0.004). There was no correlation between secreted protein acidic and rich in cysteine (SPARC) status and PFS in either treatment arm.
Conclusions: nab-Paclitaxel significantly improved PFS and DCR compared with dacarbazine, with a manageable safety profile.
Keywords: BRAF; chemotherapy-naïve; dacarbazine; metastatic melanoma; nab-Paclitaxel.
© The Author 2015. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Is chemotherapy still an option in the treatment of melanoma?Ann Oncol. 2015 Nov;26(11):2203-4. doi: 10.1093/annonc/mdv361. Epub 2015 Sep 15. Ann Oncol. 2015. PMID: 26374287 No abstract available.
References
-
- Boyle GM. Therapy for metastatic melanoma: an overview and update. Expert Rev Anticancer Ther 2011; 11: 725–737. - PubMed
-
- Robert C, Thomas L, Bondarenko I et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–2526. - PubMed
-
- Hauschild A, Grob JJ, Demidov LV et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380: 358–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous