Genetic code flexibility in microorganisms: novel mechanisms and impact on physiology
- PMID: 26411296
- PMCID: PMC4712924
- DOI: 10.1038/nrmicro3568
Genetic code flexibility in microorganisms: novel mechanisms and impact on physiology
Abstract
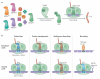
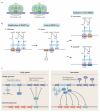
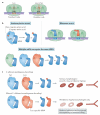
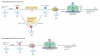
The genetic code, initially thought to be universal and immutable, is now known to contain many variations, including biased codon usage, codon reassignment, ambiguous decoding and recoding. As a result of recent advances in the areas of genome sequencing, biochemistry, bioinformatics and structural biology, our understanding of genetic code flexibility has advanced substantially in the past decade. In this Review, we highlight the prevalence, evolution and mechanistic basis of genetic code variations in microorganisms, and we discuss how this flexibility of the genetic code affects microbial physiology.
Figures

References
-
- Li M, Tzagoloff A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell. 1979;18:47–53. - PubMed
-
-
Macino G, Coruzzi G, Nobrega FG, Li M, Tzagoloff A. Use of the UGA terminator as a tryptophan codon in yeast mitochondria. Proc. Natl Acad. Sci. USA. 1979;76:3784–3785. First discovery of codon reassignment in microorganisms.
-
-
- Ambrogelly A, Palioura S, Söll D. Natural expansion of the genetic code. Nat. Chem. Biol. 2007;3:29–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

