Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism
- PMID: 26411365
- PMCID: PMC4809801
- DOI: 10.1038/onc.2015.353
Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism
Abstract
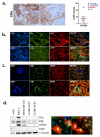
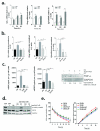
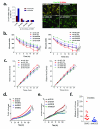
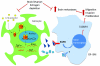
Brain metastases (BM) are a devastating consequence of breast cancer. BM occur more frequently in patients with estrogen receptor-negative (ER-) breast cancer subtypes; HER2 overexpressing (HER2+) tumors and triple-negative (TN) (ER-, progesterone receptor-negative (PR-) and normal HER2) tumors. Young age is an independent risk factor for the development of BM, thus we speculated that higher circulating estrogens in young, pre-menopausal women could exert paracrine effects through the highly estrogen-responsive brain microenvironment. Using a TN experimental metastases model, we demonstrate that ovariectomy decreased the frequency of magnetic resonance imaging-detectable lesions by 56% as compared with estrogen supplementation, and that the combination of ovariectomy and letrozole further reduced the frequency of large lesions to 14.4% of the estrogen control. Human BM expressed 4.2-48.4% ER+ stromal area, particularly ER+ astrocytes. In vitro, E2-treated astrocytes increased proliferation, migration and invasion of 231BR-EGFP cells in an ER-dependent manner. E2 upregulated epidermal growth factor receptor (EGFR) ligands Egf, Ereg and Tgfa mRNA and protein levels in astrocytes, and activated EGFR in brain metastatic cells. Co-culture of 231BR-EGFP cells with E2-treated astrocytes led to the upregulation of the metastatic mediator S100 Calcium-binding protein A4 (S100A4) (1.78-fold, P<0.05). Exogenous EGF increased S100A4 mRNA levels in 231BR-EGFP cells (1.40±0.02-fold, P<0.01 compared with vehicle control) and an EGFR/HER2 inhibitor blocked this effect, suggesting that S100A4 is a downstream effector of EGFR activation. Short hairpin RNA-mediated S100A4 silencing in 231BR-EGFP cells decreased their migration and invasion in response to E2-CM, abolished their increased proliferation in co-cultures with E2-treated astrocytes and decreased brain metastatic colonization. Thus, S100A4 is one effector of the paracrine action of E2 in brain metastatic cells. These studies provide a novel mechanism by which estrogens, acting through ER+ astrocytes in the brain microenvironment, can promote BM of TN breast cancers, and suggests existing endocrine agents may provide some clinical benefit towards reducing and managing BM.
Figures



References
-
- Cheng X, Hung MC. Breast cancer brain metastases. Cancer metastasis reviews. 2007;26(3-4):635–43. - PubMed
-
- Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(14):4834–43. - PubMed
-
- Boogerd W, Vos VW, Hart AA, Baris G. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol. 1993;15(2):165–74. - PubMed
-
- Braccini AL, Azria D, Thezenas S, Romieu G, Ferrero JM, Jacot W. Prognostic factors of brain metastases from breast cancer: Impact of targeted therapies. The Breast. 2013;22(5):993–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

