Cargo Delivery into the Brain by in vivo identified Transport Peptides
- PMID: 26411801
- PMCID: PMC4585929
- DOI: 10.1038/srep14104
Cargo Delivery into the Brain by in vivo identified Transport Peptides
Abstract
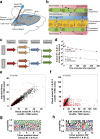
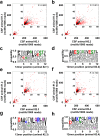
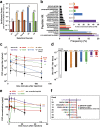
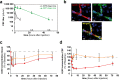
The blood-brain barrier and the blood-cerebrospinal fluid barrier prevent access of biotherapeutics to their targets in the central nervous system and therefore prohibit the effective treatment of neurological disorders. In an attempt to discover novel brain transport vectors in vivo, we injected a T7 phage peptide library and continuously collected blood and cerebrospinal fluid (CSF) using a cisterna magna cannulated conscious rat model. Specific phage clones were highly enriched in the CSF after four rounds of selection. Validation of individual peptide candidates showed CSF enrichments of greater than 1000-fold. The biological activity of peptide-mediated delivery to the brain was confirmed using a BACE1 peptide inhibitor linked to an identified novel transport peptide which led to a 40% reduction of Amyloid-β in CSF. These results indicate that the peptides identified by the in vivo phage selection approach could be useful transporters for systemically administrated large molecules into the brain with therapeutic benefits.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Johanson C. E., Duncan J. A., Stopa E. G. & Baird A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res 22, 1011–1037, 10.1007/s11095-005-6039-0 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

