cGMP-Manufactured Human Induced Pluripotent Stem Cells Are Available for Pre-clinical and Clinical Applications
- PMID: 26411904
- PMCID: PMC4624993
- DOI: 10.1016/j.stemcr.2015.08.015
cGMP-Manufactured Human Induced Pluripotent Stem Cells Are Available for Pre-clinical and Clinical Applications
Abstract
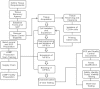
The discovery of induced pluripotent stem cells (iPSCs) and the concurrent development of protocols for their cell-type-specific differentiation have revolutionized our approach to cell therapy. It has now become critical to address the challenges related to the generation of iPSCs under current good manufacturing practice (cGMP) compliant conditions, including tissue sourcing, manufacturing, testing, and storage. Furthermore, regarding the technical challenges, it is very important to keep the costs of manufacturing and testing reasonable and solve logistic hurdles that permit the global distribution of these products. Here we describe our efforts to develop a process for the manufacturing of iPSC master cell banks (MCBs) under cGMPs and announce the availability of such banks.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Andrews P.W., Cavagnaro J., Deans R., Feigal E., Horowitz E., Keating A., Rao M., Turner M., Wilmut I., Yamanaka S. Harmonizing standards for producing clinical-grade therapies from pluripotent stem cells. Nat. Biotechnol. 2014;32:724–726. - PubMed
-
- Andrews P.W., Baker D., Benvinisty N., Miranda B., Bruce K., Brüstle O., Choi M., Choi Y.M., Crook J.M., de Sousa P.A. Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI) Regen. Med. 2015;10(2, Suppl):1–44. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

