Early Observations on the Use of Riociguat in a Large, Metropolitan Pulmonary Arterial Hypertension/Chronic Thromboembolic Pulmonary Hypertension Treatment Center
- PMID: 26411969
- PMCID: PMC4675747
- DOI: 10.1007/s40119-015-0046-y
Early Observations on the Use of Riociguat in a Large, Metropolitan Pulmonary Arterial Hypertension/Chronic Thromboembolic Pulmonary Hypertension Treatment Center
Abstract
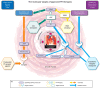
Introduction: Pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) are rare, life-threatening diseases in which chronically elevated pressure in the pulmonary arteries results in vascular remodeling and right heart failure. Treatment goals are to improve patient functioning, exercise capacity, and symptoms; delay disease progression; normalize the right ventricular function; and, ultimately, improve survival. Therapeutic management centers on the affected physiologic pathways and includes endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostacyclins. Recently, riociguat, a novel therapeutic agent that stimulates soluble guanylate cyclase via the nitric oxide pathway, was approved for the treatment of both PAH and CTEPH. Clinical trial data show that riociguat significantly improves exercise capacity as well as hemodynamic parameters in PAH/CTEPH.
Methods: We report on the early use of riociguat at our center-a large, metropolitan pulmonary hypertension treatment facility that cares for >250 patients with PAH/CTEPH. Through our initial clinical experience, we offer evidence on the benefits of riociguat in three patients with PAH associated with different etiologies, symptoms, and treatment goals.
Results: Overall, patients at our center who have received riociguat have experienced clinical benefits, including improvement in symptomatic and hemodynamic parameters, increase in 6-min walk distance, and improvement or stabilization of World Health Organization functional class. In several cases, initial response to riociguat has been encouraging and has helped patients reach their treatment goals. Riociguat appears to be well tolerated, with only one patient experiencing mild, self-limiting side effects.
Conclusion: Novel agents are continuously being introduced into the PAH/CTEPH armamentarium, and clinicians must decide how best to integrate them into their existing treatment algorithms. This case series offers initial evidence from our practice on the benefits of riociguat in optimizing hemodynamic and functional parameters. These benefits have been observed in PAH associated with different etiologies and functional status, and in both first-line and combination use.
Funding: Bayer HealthCare Pharmaceuticals.
Keywords: Chronic thromboembolic pulmonary hypertension (CTEPH); Nitric oxide pathway; Pulmonary arterial hypertension (PAH); Riociguat; Soluble guanylate cyclase.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

