Copper-free click chemistry for attachment of biomolecules in magnetic tweezers
- PMID: 26413268
- PMCID: PMC4582843
- DOI: 10.1186/s13628-015-0023-9
Copper-free click chemistry for attachment of biomolecules in magnetic tweezers
Abstract
Background: Single-molecule techniques have proven to be an excellent approach for quantitatively studying DNA-protein interactions at the single-molecule level. In magnetic tweezers, a force is applied to a biopolymer that is anchored between a glass surface and a magnetic bead. Whereas the relevant force regime for many biological processes is above 20pN, problems arise at these higher forces, since the molecule of interest can detach from the attachment points at the surface or the bead. Whereas many recipes for attachment of biopolymers have been developed, most methods do not suffice, as the molecules break at high force, or the attachment chemistry leads to nonspecific cross reactions with proteins.
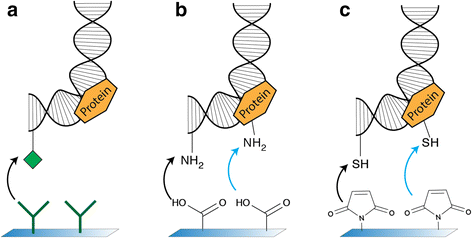
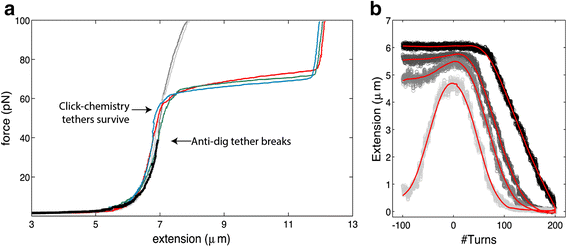
Results: Here, we demonstrate a novel attachment method using copper-free click chemistry, where a DBCO-tagged DNA molecule is bound to an azide-functionalized surface. We use this new technique to covalently attach DNA to a flow cell surface. We show that this technique results in covalently linked tethers that are torsionally constrained and withstand very high forces (>100pN) in magnetic tweezers.
Conclusions: This novel anchoring strategy using copper-free click chemistry allows to specifically and covalently link biomolecules, and conduct high-force single-molecule experiments. Excitingly, this advance opens up the possibility for single-molecule experiments on DNA-protein complexes and molecules that are taken directly from cell lysate.
Keywords: Copper-free click chemistry; DNA immobilization; Magnetic tweezers; SPAAC reactions; Surface chemistry.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

