Outcomes after viral load rebound on first-line antiretroviraltreatment in children with HIV in the UK and Ireland: an observational cohort study
- PMID: 26413561
- PMCID: PMC4580991
- DOI: 10.1016/S2352-3018(15)00021-1
Outcomes after viral load rebound on first-line antiretroviraltreatment in children with HIV in the UK and Ireland: an observational cohort study
Abstract
Background: About a third of children with HIV have virological failure within 2 years of beginning antiretroviral treatment (ART). We assessed the probability of switch to second-line ART or virological re-suppression without switch in children who had virological rebound on first-line ART in the UK and Ireland.
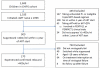
Methods: In this study, we used data reported to the Collaborative HIV Paediatric Study (CHIPS), a national multicentre observational cohort. We included children with virological rebound (confirmed viral load>400 copies per mL after suppression<400 copies per mL) on first-line ART. We did a competing-risk analysis to estimate the probability of switch to second-line treatment, confirmed resuppression (two consecutive viral load measurments<400 copies per mL) without switch, and continued viral load above 400 copies per mL without switch. We also assessed factors that predicted a faster time to switch.
Findings: Of the 900 children starting first-line ART who had a viral load below 400 copies per mL within a year of starting treatment, 170 (19%) had virological rebound by a median of 20·6 months (IQR 9·7–40·5). At rebound, median age was 10·6 years (5·6–13·4), median viral load was 3·6 log10 copies per mL (3·1–4·2), and median CD4% was 24% (17–32). 89 patients (52%) switched to second-line ART at a median of 4·9 months (1·7–13·4) after virological rebound, 53 (31%) resuppressed without switch (19 [61%] of 31 patients on a first-line regimen that included a protease inhibitor and 31 [24%] of 127 patients on a first-line regimen that included a non-nucleoside reverse transcriptase inhibitor; NNRTI), and 28 (16%) neither resuppressed nor switched. At 12 months after rebound, the estimated probability of switch was 38% (95% CI 30–45) and of resuppression was 27% (21–34). Faster time to switch was associated with a higher viral load (p<0·0001), later calendar year at virological rebound (p=0·02), and being on an NNRTI-based or triple nucleoside reverse transcriptase inhibitor-based versus protease-inhibitor-based first-line regimen (p=0·001).
Interpretation: A third of children with virological rebound resuppressed without switch. Clinicians should consider the possibility of resuppression with adherence support before switching treatment in children with HIV.
Funding: NHS England (London Specialised Commissioning Group).
Figures
Comment in
-
Maintenance of antiretroviral efficacy in children.Lancet HIV. 2015 Apr;2(4):e120-1. doi: 10.1016/S2352-3018(15)00040-5. Epub 2015 Mar 17. Lancet HIV. 2015. PMID: 26424670 No abstract available.
References
-
- Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving antiretroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. The Pediatric infectious disease journal. 2011;30(1):52–6. - PubMed
-
- World Health Organization . Consolidated guidelines on general HIV care and the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials