The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: A different point of view on the role of aquaporins
- PMID: 26413835
- PMCID: PMC4905710
- DOI: 10.1002/glia.22921
The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: A different point of view on the role of aquaporins
Abstract
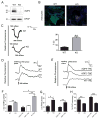
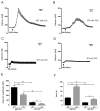
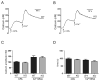
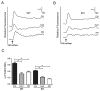
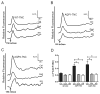
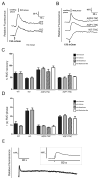
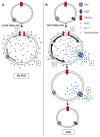
Regulatory volume decrease (RVD) is a process by which cells restore their original volume in response to swelling. In this study, we have focused on the role played by two different Aquaporins (AQPs), Aquaporin-4 (AQP4), and Aquaporin-1 (AQP1), in triggering RVD and in mediating calcium signaling in astrocytes under hypotonic stimulus. Using biophysical techniques to measure water flux through the plasma membrane of wild-type (WT) and AQP4 knockout (KO) astrocytes and of an astrocyte cell line (DI TNC1) transfected with AQP4 or AQP1, we here show that AQP-mediated fast swelling kinetics play a key role in triggering and accelerating RVD. Using calcium imaging, we show that AQP-mediated fast swelling kinetics also significantly increases the amplitude of calcium transients inhibited by Gadolinium and Ruthenium Red, two inhibitors of the transient receptor potential vanilloid 4 (TRPV4) channels, and prevented by removing extracellular calcium. Finally, inhibition of TRPV4 or removal of extracellular calcium does not affect RVD. All together our study provides evidence that (1) AQP influenced swelling kinetics is the main trigger for RVD and in mediating calcium signaling after hypotonic stimulus together with TRPV4, and (2) calcium influx from the extracellular space and/or TRPV4 are not essential for RVD to occur in astrocytes.
Keywords: AQP1; AQP4; RVD; cell swelling; hypotonic stimulus.
© 2015 Wiley Periodicals, Inc.
Figures
References
-
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. Review. - PubMed
-
- Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004;18:542–544. - PubMed
-
- Basco D, Blaauw B, Pisani F, Sparaneo A, Nicchia GP, Mola MG, Reggiani C, Svelto M, Frigeri A. AQP4-dependent water transport plays a functional role in exercise-induced skeletal muscle adaptations. PLoS One. 2013;8:e58712. doi: 10.1371/journal.pone.0058712. Erratum in: PLoS One 8(6). doi:10.1371/annotation/86fc2632-913c-490d-8b9b-e925b38baec5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

