TIGIT predominantly regulates the immune response via regulatory T cells
- PMID: 26413872
- PMCID: PMC4639980
- DOI: 10.1172/JCI81187
TIGIT predominantly regulates the immune response via regulatory T cells
Retraction in
-
TIGIT predominantly regulates the immune response via regulatory T cells.J Clin Invest. 2024 Jul 1;134(13):e183278. doi: 10.1172/JCI183278. J Clin Invest. 2024. PMID: 38949028 Free PMC article. No abstract available.
Abstract
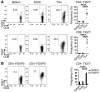
Coinhibitory receptors are critical for the maintenance of immune homeostasis. Upregulation of these receptors on effector T cells terminates T cell responses, while their expression on Tregs promotes their suppressor function. Understanding the function of coinhibitory receptors in effector T cells and Tregs is crucial, as therapies that target coinhibitory receptors are currently at the forefront of treatment strategies for cancer and other chronic diseases. T cell Ig and ITIM domain (TIGIT) is a recently identified coinhibitory receptor that is found on the surface of a variety of lymphoid cells, and its role in immune regulation is just beginning to be elucidated. We examined TIGIT-mediated immune regulation in different murine cancer models and determined that TIGIT marks the most dysfunctional subset of CD8+ T cells in tumor tissue as well as tumor-tissue Tregs with a highly active and suppressive phenotype. We demonstrated that TIGIT signaling in Tregs directs their phenotype and that TIGIT primarily suppresses antitumor immunity via Tregs and not CD8+ T cells. Moreover, TIGIT+ Tregs upregulated expression of the coinhibitory receptor TIM-3 in tumor tissue, and TIM-3 and TIGIT synergized to suppress antitumor immune responses. Our findings provide mechanistic insight into how TIGIT regulates immune responses in chronic disease settings.
Figures
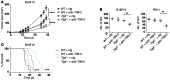
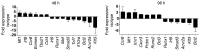




References
-
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

