Anti-Aging and Tissue Regeneration Ability of Policosanol Along with Lipid-Lowering Effect in Hyperlipidemic Zebrafish via Enhancement of High-Density Lipoprotein Functionality
- PMID: 26413884
- PMCID: PMC4841090
- DOI: 10.1089/rej.2015.1745
Anti-Aging and Tissue Regeneration Ability of Policosanol Along with Lipid-Lowering Effect in Hyperlipidemic Zebrafish via Enhancement of High-Density Lipoprotein Functionality
Abstract
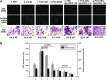
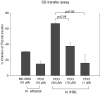
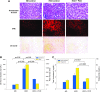
We investigated the tissue regeneration and lipid-lowering effects of policosanol (PCO) by employing a hyperlipidemic zebrafish model. A reconstituted high-density lipoprotein containing policosanol (PCO-rHDL) facilitated greater cell growth and replication with less apoptosis and reactive oxygen species (ROS) production in BV-2 microglial cell lines. From in vivo study, injection of rHDL containing apolipoprotein A-I (ApoA-I) caused 76 ± 4% (p = 0.01) greater tissue regeneration activity than the phosphate-buffered saline (PBS) control, whereas PCO-rHDL caused 94 ± 7% (p = 0.002) increased regeneration. PCO in ethanol (EtOH) showed lower cholesteryl ester transfer protein (CETP) inhibitory ability than did anacetrapib, whereas PCO-rHDL showed higher inhibitory ability than anacetrapib, suggesting a synergistic effect between PCO and rHDL. Following 9 weeks of PCO consumption, the PCO group (0.003% PCO in Tetrabit) showed the highest survivability (80%), whereas normal diet (ND) and high-cholesterol diet (HCD) control groups showed 67% and 70% survival rates, respectively. Supplementation with a HCD resulted in two-fold elevation of CETP activity along with 3- and 2.5-fold increases in serum total cholesterol (TC) and triglycerides (TGs) levels, respectively. Consumption of PCO for 9 weeks resulted in 40 ± 5% (p = 0.01 vs. HCD) and 33 ± 4% (p = 0.02 vs. HCD) reduction of TC and TGs levels, respectively. Serum high-density lipoprotein cholesterol (HDL-C) level increased up to 37 ± 2 mg/dL (p = 0.004), whereas the percentage of HDL-C/TC increased up to 20 ± 2% from 5 ± 1% compared to the HCD control. The serum glucose level was reduced to 47 ± 2% (p = 0.002) compared to the HCD control. Fatty liver change and hepatic inflammation levels were remarkably increased upon HCD consumption and were two-fold higher than that under ND. However, the PCO group showed 58 ± 5% (p = 0.001) and 50 ± 3% (p = 0.006) reduction of inflammation enzyme levels and lipid content in hepatic tissue under HCD. In conclusion, PCO supplementation showed lipid-lowering and HDL-C-elevating effects with ameliorating fatty liver change. These in vivo anti-atherosclerotic and anti-diabetic effects of PCO are well associated with in vitro anti-apoptotic activities.
Figures


References
-
- Wang YW, Jones PJ, Pischel I, Fairow C. Effects of policosanols and phytosterols on lipid levels and cholesterol biosynthesis in hamsters. Lipids 2003;38:165–170 - PubMed
-
- Menéndez R, Arruzazabala L, Más R, Del Río A, Amor AM, González RM, Carbajal D, Fraga V, Molina V, Illnait J. Cholesterol-lowering effect of policosanol on rabbits with hypercholesterolaemia induced by a wheat starch-casein diet. Br J Nutr 1997;77:923–932 - PubMed
-
- Arruzazabala ML, Carbajal D, Mas R, Molina V, Valdes S, Laguna A. Cholesterol-lowering effects of policosanol in rabbits. Biol Res 1994;27:205–208 - PubMed
-
- Rodríguez-Echenique C, Mesa R, Más R, Noa M, Menéndez R, González RM, Amor AM, Fraga V, Sotolongo V, Laguna A. Effects of policosanol chronically administered in male monkeys (Macaca arctoides). Food Chem Toxicol 1994;32:565–575 - PubMed
-
- Noa M, de la Rosa MC, Más R. Effect of policosanol on foam-cell formation in carrageenan-induced granulomas in rats. J Pharm Pharmacol 1996;48:306–309 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
