Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity
- PMID: 26414765
- PMCID: PMC4618176
- DOI: 10.1038/ni.3268
Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity
Abstract
Mitochondria need to be juxtaposed to phagosomes for the synergistic production of ample reactive oxygen species (ROS) in phagocytes to kill pathogens. However, how phagosomes transmit signals to recruit mitochondria has remained unclear. Here we found that the kinases Mst1 and Mst2 functioned to control ROS production by regulating mitochondrial trafficking and mitochondrion-phagosome juxtaposition. Mst1 and Mst2 activated the GTPase Rac to promote Toll-like receptor (TLR)-triggered assembly of the TRAF6-ECSIT complex that is required for the recruitment of mitochondria to phagosomes. Inactive forms of Rac, including the human Rac2(D57N) mutant, disrupted the TRAF6-ECSIT complex by sequestering TRAF6 and substantially diminished ROS production and enhanced susceptibility to bacterial infection. Our findings demonstrate that the TLR-Mst1-Mst2-Rac signaling axis is critical for effective phagosome-mitochondrion function and bactericidal activity.
Figures

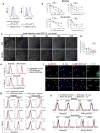
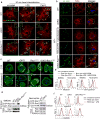

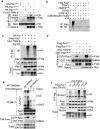

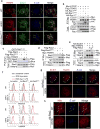
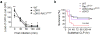
Comment in
-
De-Mst-ifying microbicidal killing.Nat Immunol. 2015 Nov;16(11):1107-8. doi: 10.1038/ni.3291. Nat Immunol. 2015. PMID: 26482969 No abstract available.
-
Phagocytosis: Mitochondria and phagosomes: better together.Nat Rev Immunol. 2015 Nov;15(11):667. doi: 10.1038/nri3931. Nat Rev Immunol. 2015. PMID: 26494048 No abstract available.
References
-
- West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annual review of cell and developmental biology. 2006;22:409–437. - PubMed
-
- Beutler B, et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annual review of immunology. 2006;24:353–389. - PubMed
-
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nature reviews Immunology. 2004;4:181–189. - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews. 2007;87:245–313. - PubMed
-
- Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100:2692–2696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

