In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks
- PMID: 26414863
- PMCID: PMC4722541
- DOI: 10.1089/ten.TEC.2015.0239
In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks
Abstract
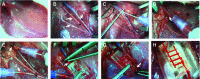
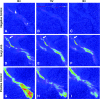
The field of tissue engineering has advanced the development of increasingly biocompatible materials to mimic the extracellular matrix of vascularized tissue. However, a majority of studies instead rely on a multiday inosculation between engineered vessels and host vasculature rather than the direct connection of engineered microvascular networks with host vasculature. We have previously demonstrated that the rapid casting of three-dimensionally-printed (3D) sacrificial carbohydrate glass is an expeditious and a reliable method of creating scaffolds with 3D microvessel networks. Here, we describe a new surgical technique to directly connect host femoral arteries to patterned microvessel networks. Vessel networks were connected in vivo in a rat femoral artery graft model. We utilized laser Doppler imaging to monitor hind limb ischemia for several hours after implantation and thus measured the vascular patency of implants that were anastomosed to the femoral artery. This study may provide a method to overcome the challenge of rapid oxygen and nutrient delivery to engineered vascularized tissues implanted in vivo.
Figures

References
-
- Lee A.Y., Mahler N., Best C., Lee Y.U., and Breuer C.K. Regenerative implants for cardiovascular tissue engineering., Transl Res 163, 321, 2014 - PubMed
-
- Nugent H.M., and Edelman E.R. Tissue engineering therapy for cardiovascular disease. Circ Res 92, 1068, 2003 - PubMed
-
- O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater Today 14, 88, 2011
-
- Radhakrishnan J., Krishnan U.M., and Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv 32, 449, 2014 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

