Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066)
- PMID: 26414912
- PMCID: PMC4692123
- DOI: 10.1089/AID.2015.0182
Dose Frequency Ranging Pharmacokinetic Study of Tenofovir-Emtricitabine After Directly Observed Dosing in Healthy Volunteers to Establish Adherence Benchmarks (HPTN 066)
Abstract
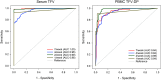
Oral preexposure prophylaxis (PrEP) trials report disparate efficacy attributed to variable adherence. HPTN 066 was conducted to establish objective, quantitative benchmarks for discrete, regular levels of adherence using directly observed dosing of tenofovir (TFV) disoproxil fumarate (TDF)/emtricitabine (FTC). Healthy, HIV-uninfected men and women were randomized to one of four oral regimens of fixed-dose TDF 300 mg/FTC 200 mg tablet for 5 weeks with all doses observed: one tablet weekly (one/week), one tablet twice weekly (two/week), two tablets twice weekly (four/week), or one tablet daily (seven/week). Trough serum TFV and FTC, peripheral blood mononuclear cell (PBMC), and CD4(+) TFV-diphosphate (TFV-DP) and FTC-triphosphate (FTC-TP) concentrations were determined throughout dosing and 2 weeks after the last dose. Rectosigmoidal, semen, and cervicovaginal samples were collected for drug assessment at end of dosing and 2 weeks later in a subset of participants. The 49 enrolled participants tolerated the regimens well. All regimens achieved steady-state concentrations by the second dose for serum TFV/FTC and by 7 days for PBMC TFV-DP/FTC-TP. Steady-state median TFV-DP predose concentrations demonstrated dose proportionality: one/week 1.6 fmol/10(6) PBMCs, two/week 9.1, four/week 18.8, seven/week, 36.3. Further, TFV-DP was consistently quantifiable 2 weeks after the last dose for the ≥4/week regimens. Adherence benchmarks were identified using receiver operating characteristic curves, which had areas under the curve ≥0.93 for all analytes in serum and PBMCs. Intersubject and intrasubject coefficients of variation (%CV) ranged from 33% to 63% and 14% to 34%, respectively, for all analytes in serum and PBMCs. Steady-state PBMC TFV-DP was established earlier and at lower concentrations than predicted and was the only analyte demonstrating predose concentration dose proportionality. Steady-state daily dosing serum TFV and PBMC TFV-DP was consistent with highly effective PrEP clinical trials. HPTN 066 provides adherence benchmarks for oral TFV/FTC regimens to assist interpreting study outcomes.
Figures



References
-
- Choopanya K, Martin M, Suntharasamai P, et al. : Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381:2083–2090 - PubMed
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. : Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–434 - PubMed
-
- Marrazzo JM, Ramjee G, Richardson B, et al. : Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). New Engl J Med 2015;372:509–518 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- P30AI094189/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- P30AI50410/AI/NIAID NIH HHS/United States
- T32 DK007632/DK/NIDDK NIH HHS/United States
- UL1TR001079/TR/NCATS NIH HHS/United States
- UM1AI069465/AI/NIAID NIH HHS/United States
- T32 GM066691/GM/NIGMS NIH HHS/United States
- UM1 AI068613/AI/NIAID NIH HHS/United States
- UM1AI068613/AI/NIAID NIH HHS/United States
- UM1AI069423/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

