The Effects of Milnacipran on Sleep Disturbance in Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study
- PMID: 26414990
- PMCID: PMC4702190
- DOI: 10.5664/jcsm.5400
The Effects of Milnacipran on Sleep Disturbance in Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled, Two-Way Crossover Study
Abstract
Objective: This study examined the effects of milnacipran on polysomnographic (PSG) measures of sleep and subjective complaints in patients with fibromyalgia and disturbed sleep.
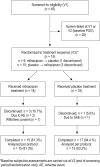
Methods: This was a single-site, double-blind, placebo-controlled, two-period crossover PSG study. Eligible subjects (aged 28-72 y) were randomized (1:1) to milnacipran (100 mg/d) or placebo for crossover period 1, and vice versa for period 2. Each crossover period comprised a dose-escalation and dose-maintenance phase, with a 2-w taper/washout between periods. In-laboratory PSGs were collected at baseline, and at the end of each treatment period. The primary endpoints were the difference in PSG-recorded wake after sleep onset (WASO), number of awakenings after sleep onset (NAASO), and sleep efficiency (SE) between 4 w of maintenance treatment with milnacipran and placebo. Other PSG measures, subject-rated sleep, fatigue, physical functioning, and pain were assessed. Post hoc analysis was performed in subjects showing at least 25% reduction in pain from baseline in the Brief Pain Inventory Score (responders).
Results: Of 19 subjects randomized, 15 completed both periods. Subjects treated with milnacipran showed no significant improvements in WASO and NAASO, but showed reduced SE (p = 0.049). Milnacipran did not show significant improvement in other PSG parameters or subjective endpoints. Two thirds of completers met responder criteria and additionally showed a significant improvement in daily effect of pain (p = 0.043) and subjective sleep quality (p = 0.040).
Conclusion: The data suggest that milnacipran is not sedating in most patients with fibromyalgia and improvements in sleep are likely a result of pain improvement.
Clinical trial registration: ClinicalTrials.gov, identifier: NCT01234675.
Keywords: fibromyalgia; milnacipran; pain; polysomnography; sleep quality.
© 2016 American Academy of Sleep Medicine.
Figures
References
-
- Wolfe F, Ross K, Anderson J, Russel IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. - PubMed
-
- Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–8. - PubMed
-
- Landis CA, Frey CA, Lentz MJ, Rethernel J, Buchwald D, Shaver JLF. Self reported sleep quality and fatigue correlates with actigraphy sleep indicators in midlife women with fibromyalgia. Nurs Res. 2003;52:140–7. - PubMed
-
- Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. - PubMed
-
- Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous