Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2
- PMID: 26415026
- PMCID: PMC4684781
- DOI: 10.1016/j.freeradbiomed.2015.08.009
Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2
Erratum in
-
Corrigendum to ''Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2'' [Free Radic. Biol. Med. 89 (2015) 431-42].Free Radic Biol Med. 2016 Aug;97:621. doi: 10.1016/j.freeradbiomed.2016.06.022. Epub 2016 Jul 22. Free Radic Biol Med. 2016. PMID: 27460838 Free PMC article. No abstract available.
Abstract
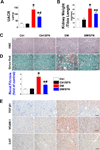
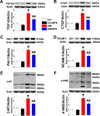
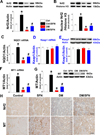
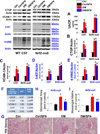
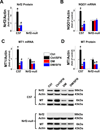
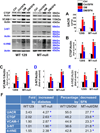
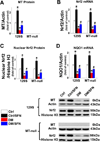
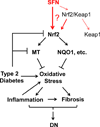
Sulforaphane (SFN) prevents diabetic nephropathy (DN) in type 1 diabetes via up-regulation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). However, it has not been addressed whether SFN also prevents DN from type 2 diabetes or which Nrf2 downstream gene(s) play(s) the key role in SFN renal protection. Here we investigated whether Nrf2 is required for SFN protection against type 2 diabetes-induced DN and whether metallothionein (MT) is an Nrf2 downstream antioxidant using Nrf2 knockout (Nrf2-null) mice. In addition, MT knockout mice were used to further verify if MT is indispensable for SFN protection against DN. Diabetes-increased albuminuria, renal fibrosis, and inflammation were significantly prevented by SFN, and Nrf2 and MT expression was increased. However, SFN renal protection was completely lost in Nrf2-null diabetic mice, confirming the pivotal role of Nrf2 in SFN protection from type 2 diabetes-induced DN. Moreover, SFN failed to up-regulate MT in the absence of Nrf2, suggesting that MT is an Nrf2 downstream antioxidant. MT deletion resulted in a partial, but significant attenuation of SFN renal protection from type 2 diabetes, demonstrating a partial requirement for MT for SFN renal protection. Therefore, the present study demonstrates for the first time that as an Nrf2 downstream antioxidant, MT plays an important, though partial, role in mediating SFN renal protection from type 2 diabetes.
Keywords: Diabetes; Fibrosis; Inflammation; Kidney; Oxidative stress.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Metallothionein Is Downstream of Nrf2 and Partially Mediates Sulforaphane Prevention of Diabetic Cardiomyopathy.Diabetes. 2017 Feb;66(2):529-542. doi: 10.2337/db15-1274. Epub 2016 Nov 30. Diabetes. 2017. PMID: 27903744 Free PMC article.
-
Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function.Clin Sci (Lond). 2020 Sep 30;134(18):2469-2487. doi: 10.1042/CS20191088. Clin Sci (Lond). 2020. PMID: 32940670
-
Nrf2 expression and function, but not MT expression, is indispensable for sulforaphane-mediated protection against intermittent hypoxia-induced cardiomyopathy in mice.Redox Biol. 2018 Oct;19:11-21. doi: 10.1016/j.redox.2018.07.014. Epub 2018 Jul 21. Redox Biol. 2018. PMID: 30096613 Free PMC article.
-
The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy.Toxicol Mech Methods. 2013 Jan;23(1):27-33. doi: 10.3109/15376516.2012.735277. Epub 2012 Nov 29. Toxicol Mech Methods. 2013. PMID: 23039870 Review.
-
Role of Nuclear Factor Erythroid 2-Related Factor 2 in Diabetic Nephropathy.J Diabetes Res. 2017;2017:3797802. doi: 10.1155/2017/3797802. Epub 2017 Apr 23. J Diabetes Res. 2017. PMID: 28512642 Free PMC article. Review.
Cited by
-
Clopidogrel Reduces Fibronectin Accumulation and Improves Diabetes-Induced Renal Fibrosis.Int J Biol Sci. 2019 Jan 1;15(1):239-252. doi: 10.7150/ijbs.29063. eCollection 2019. Int J Biol Sci. 2019. PMID: 30662363 Free PMC article.
-
The Role of HSP90α in Methamphetamine/Hyperthermia-Induced Necroptosis in Rat Striatal Neurons.Front Pharmacol. 2021 Jul 19;12:716394. doi: 10.3389/fphar.2021.716394. eCollection 2021. Front Pharmacol. 2021. PMID: 34349659 Free PMC article.
-
Invited Perspective: New Insight into Cadmium-Related Osteoporosis Yields Hope for Prevention and Therapy.Environ Health Perspect. 2024 Jun;132(6):61301. doi: 10.1289/EHP15263. Epub 2024 Jun 19. Environ Health Perspect. 2024. PMID: 38896781 Free PMC article. No abstract available.
-
Physiological activities of the combination of fish oil and α-lipoic acid affecting hepatic lipogenesis and parameters related to oxidative stress in rats.Eur J Nutr. 2018 Jun;57(4):1545-1561. doi: 10.1007/s00394-017-1440-0. Epub 2017 Mar 20. Eur J Nutr. 2018. PMID: 28321544
-
Protective role of NRF2 in macrovascular complications of diabetes.J Cell Mol Med. 2020 Aug;24(16):8903-8917. doi: 10.1111/jcmm.15583. Epub 2020 Jul 6. J Cell Mol Med. 2020. PMID: 32628815 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

